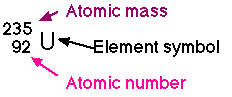Lesson 1: Elements and Atoms
5. Atomic Structure and Ions
Atomic Structure and Ions
Our present knowledge of matter results from the work of numerous people over several centuries. With thoughtful observation, these people built on the discoveries of others to reveal how substances behave. They compared their research and gradually developed more exact methods of testing new ideas. Models or concepts which could explain experimental results and predict future findings often became accepted theories. But with each discovery, these theories were challenged. Often the theories were rejected or greatly modified.
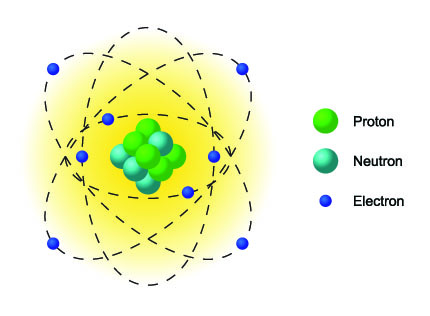
An atom has a specific structure consisting of three parts:
- Proton: symbol p+
Positively charged particle located in the center of an atom which makes up a larger portion of the mass of an atom - Neutron: symbol no
Neutral-charged particle located in the center of the atom. It makes up a large portion of the mass of the atom. - Electron: symbol e-
Negatively charged particle surrounding an atom. It has very little mass and moves about the nucleus in an electron cloud.
Calculating Numbers of Subatomic Particles:
Protons = Atomic Number
Electrons = Atomic Number( in a neutral atom)
Neutrons = Atomic mass - Atomic number
Example: Lithium has atomic number 3 so it has 3 protons and 3 electrons. It has an atomic mass of 6.79. We round this off to the nearest whole number, 7, since we cannot have a part of a proton nor neutron. So, there are 4 neutrons ( 7-3=4).
Isotopes:
Neutrons in some elements may vary. Uranium, for example, may gain or lose neutrons to form isotopes of uranium, each having a different mass.
U235 is a light isotope having 3 fewer neutrons than the most common form of uranium. U239 is a heavy isotope having 1 more neutron than the most common form.
Key Concepts
proton
neutron
electron
isotopes
|
-subatomic particle |
-relative charge |
-relative mass(amu) |
|---|---|---|
| p+ | 1+ | 1836.12 |
| no | 0 | 1838.65 |
| e- | 1- | 1 |