Lesson 3: Chemical Reactions and Equations
4. Chemical Amount: the Mole
The mole is a unit representing a certain number of things. Much the same as a dozen or a gross, but instead of being 12 or 144 things, a mole is 6.02 x 1023 things.
It is sometimes referred to as Avogadro's number and it is defined as the number of atoms in exactly 12 grams of Carbon-12. The mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is assigned a unit called atomic mass units (amu). In order to make more sensible, we use amu as gram/ mol.

The mathematical relationship between mol, molar mass and mass can be expressed as
![]()
n: mol
m: mass (gram)
M: molar mass (gram/mol)
Using the periodic table, we can calculate the molar mass (mass of 1 mol) of the H2O molecule.
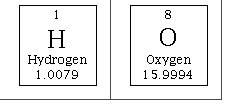
| 2H= 2 mol x 1.01 g/mol | = 2.02 g/mol |
|---|---|
| 1 O= 1 mol x16. g/mol | = 16.00 g/mol |
| 1 HOH | = 18.02 g/mol |
This brings us to the four steps that help us calculating a substance's molecular weight.
- Determine how many atoms of each different element are in the formula.
- Look up the atomic weight of each element in the periodic table
- Add the results of step three together and round off as necessary.
Calculate the molar mass of CuSO4•5H2O
Solution:
Atomic mass of Cu + S+ 4O + 5(2H +O) = 249.68 g/mol
Example 2:
Calculate the number of mol found in 10.0 g of NaCl.
Solution:
Step 1. Atomic mass of Na= 22.9898, Cl= 35.4527 g/mol (again, found on the periodic table)
Step 2. The molar mass of NaCl= 22.9898+35.4527 g/mol= 58.4425 g/ mol
Step 3. If 58.4425 g of NaCl is 1 mol, then 10.0 g will be x mol.
x= 10g/58.4425 g/mol= 0.1711 mol.
Example 3:
In gram, calculate the mass of 0.50 mol of H2O.
Solution:
Using the formula,
![]()
m= n x M
m= 0.50 mol x 18.0152 g/mol
m= 9.0076 g