Lesson 4: Chemical Bonding and Diversity of Matter
4. Intramolecular Forces
Intermolecular forces are the forces between molecules that attract one molecule to another. Without them, all substances you see around you would be in gases at all temperatures.
Van Der Waals Forces
These bonds include weak interactions between molecules. They are grouped under two major categories: London dispersion forces and dipole-dipole forces
a) London Dispersion forces are the weakest intermolecular force as they are a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms from temporary dipoles. Due to these forces that nonpolar substances condense to liquids and freeze into solids when the temperature is lowered.
The constant motion of electrons in an atom or molecule can develop instantaneous dipole when electrons distributed unsymmetrically around the nucleus.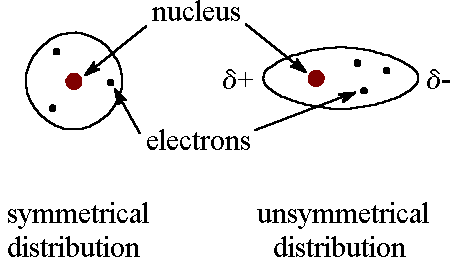
This, in turn, distorts the distribution of electrons in the second atom due to an electrostatic attraction between the + and + charges.

Dispersion forces are present between all molecules. The attraction results from this force depend on the number of electrons around the atoms involved. For example, the boiling points of the noble gases increase as one goes from He to Rn. Because larger atoms and molecules show stronger dispersion forces than smaller ones. In larger atoms, valence electrons are farther from the nuclei than in the smaller atoms or molecules. Therefore, there less tightly held and care more easily form temporary dipoles.
In non-polar molecular compounds, it is the shape of the molecule that determines the strength of the London forces. Ex. pentane (C5H12) can exist in several forms. When it is linear its boiling point is 36oC whereas it is 9.5oC when it is spherical due to the fact that linear allows for London interactions.
b) Dipole-dipole Forces: Permanent as they are, their strength depends on the electronegativity of the atoms that make up the molecule and the geometry of the molecule. These forces are due to the attraction of the positive end of one molecule to the negative end of the neighboring molecules. For example, H2O molecules have a very strong dipole because of the highly electronegative O atom compared to the H atom, and the bent shape of the molecule. CO2, on the other hand, has no dipole because the molecule is linear and the individual C-O dipoles cancel each other.
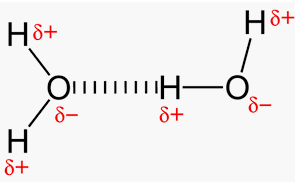
Hydrogen Bonds
Hydrogen bonds are attractive forces between a H atom covalently bonded to F, O or N in one molecule and F, O, or N of another molecule. The main factor is that F, O, and N atoms have the highest electronegativities of all the elements, so any covalent bonds between H and these elements literally leave H with a high positive charge.
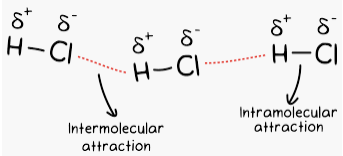
Hydrogen bonding can be intramolecular or intermolecular. Hydrogen bonding explains the high boiling point of H2O compared to those of H2S and H2Se. For example, it gives strength to various polymers (nylon, cellulose, etc) and the complementary base pairs of the DNA strands.