Lesson 4: Chemical Bonding and Diversity of Matter
6. Ionic Bonding vs. Covalent Bonding
When electrons are shared, in most cases, they are not shared equally. Some atoms have a greater attraction for the shared valence electrons than the others. This relative attraction is known as electronegativity. When two H atoms have the same electronegativity and share a pair of electrons equally between them, this is called a nonpolar covalent bond.
The difference in the electronegativity of Chlorine (3.0) and Hydrogen (2.2) means that the pair of electrons in an H-Cl bond will not be shared equally. The higher electronegativity of Cl means that the electrons will stay closer to it; making the covalent bond have one end that is partially negative, and the other end partially positive. This is called a polar covalent bond.
However, sodium with an electronegativity of 0.9 and chlorine with an electronegativity of 3.0 could react. In this case, sharing would not occur at all. Instead, Na simple transfer electrons to Cl, forming Na+ and Cl- ions. The bond between Na+ and Cl- is an ionic bond.
It is important to note that there is not a sharp dividing line between ionic bonding and covalent boing. Bonding becomes more ionic and less covalent with increased electronegativity difference. So, it is safe to state that bonding between nonmetals is referred to as covalent bonding, and bonding between metals and non-metals is referred to as ionic bonding.
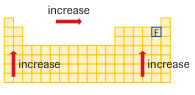 The diagram shows the electronegativity trend on the periodic table. Using the diagram above, can you identify the element with the highest and lowest electronegativity?
The diagram shows the electronegativity trend on the periodic table. Using the diagram above, can you identify the element with the highest and lowest electronegativity?