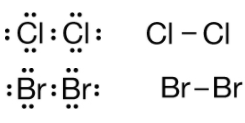Lesson 4: Chemical Bonding and Diversity of Matter
7. Self-Check Assessment
Title
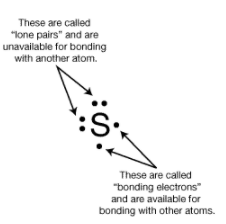
The group number provides information about the number of valence electrons. Elements in group 1(1A) all have one valence electron. 2 lone pairs and 2 bonding e-.
They all have 6 valence electrons.
SC 2. VSEPR and Polarity
SiO2: Elements that are non-metals favour covalent over ionic bond formation. The difference between their electronegativity high enough to create bonds dipole so the molecule is polar. Ni2O4: ionic compounds contain ions when
difference between electronegativity relatively big. In nickel(1V) oxide, the electrons lost by the nickel in forming N4+ have been gained by the oxygen to form O2-.