Lesson 1. Solutions and Molarity
3. Molarity
Molarity (molar concentration) is a measure of the concentration of a chemical species in a solution in terms of moles per liter of solution. Molarity can be calculated as 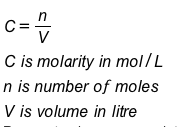
Although molarity is the most common way of expressing concentration, there are other ways as well. However, the units for C, n, and V might change depending on which way is being used. See the example problems below.
Volume-volume percent (% v/v): Vinegar is sold as percent per volume. That is a number of mL per 100 mL of solution. A typical vinegar solution is 5% acetic acid by volume. This means there is 5 mL of acetic acid for every 100 mL of vinegar.
Unit: mL/100mL
Mass-volume percentage (%w/v): Hydrogen peroxide, used for cuts and burns on the skin, is often sold as 3% w/v. This means that there are 3g of solute (hydrogen peroxide) for every 100 mL of solution.
Unit: g/100 mL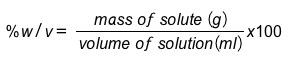
Parts per million (ppm): extremely diluted solutions often state concentration in ppm. The number of mg per L of solution or the number g of solute per 1000 L of solution. There is 2 mg/L of chlorine in pool water.
Units: mg/L or g/kg
Example 1
|
In 130 ml cup of green tea, there is about 120 mg of caffeine. Calculate the concentration of caffeine in this tea as a percentage of weight for volume. |
 |
%w/v = (0.120 g / 130 ml)x100 = 0.09%
Examples 2
A sample of solution contains 47 ppm of lead. What amount of lead is present in 300 mL of H2O(l)
C= 47 mg/L
V= 300 mL= 0.300L
n=?
n= CV = (47 mg/ L)x(0.300L) = 14.1mg ppm.
Example 3
A sample of mouthwash is a 5.0% w/v solution of table salt. What volume can be made from 15g of salt?
C= 5 g/100mL
n= 15.0g
V=?
V= n/ C => 15 g x (5g/100mL) = 300mL