Week 3 - Properties of Gases and Liquids Explained by the Particle Model
4. Density Introduction
Density Introduction
Textbook Readings
ScienceFocus 8
Page 52 and review figures 129 and 130
or
Science in Action 8
Page 42
Background Information
Density is a term which is similar to the word crowded . A large number of people in a small space is a high density of people where as a small number of people in the same space is a small density.
Which picture has the greatest density of people? 

The same applies to molecules. A large number of molecules in a space means a large density and a small number of molecules in the same space means less density.
Which of the following substances has the largest density ? 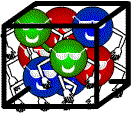

Density is related to the idea of crowded and it is also related to the idea of weight . Logically a substance with lots of molecules (more dense) is heavier then a substance with fewer molecules (less dense). Scientists do not like to use weight because it is not an accurate measurement, instead they prefer the term mass . They both measure how heavy an object is but mass is a more accurate measurement then weight. Different substances have different densities. The density of water at room temperature is 1.00 g/ml. ( g/ml = grams per milliliter). Generally, substances which are more dense then water sink , substances which are less dense then water float.
Exercise 3.4 Density Exploration (no assignment submission)
Click on the Lab and complete the assessment questions at the bottom of the lab and check your answers.
password: 9418