Weeks 27 & 28 - Humans Depend on Water Supply and Quality
Lesson 2

| Have you ever made Kool-Aid? You might have noticed that as you stirred the liquid, the sugar seemed to disappear as it dissolved into the water. You have made a solution. The water was the solvent - that part in the greater amount. The sugar is called the solute. Can you think of any other solutes in the Kool-Aid? |
 Your favorite pop is water with dissolved chemical flavoring, sugar and carbon dioxide gas. |
 |
Water is called the universal solvent because it is the most common solvent in the world. It can't dissolve petroleum based materials like gas and oil, but other inorganic materials - even some rocks can be dissolved in water! How many things can you think of that can be dissolved in water?
|
|
Other materials besides solids can dissolve in water. Dissolved gasses such as oxygen, nitrogen and carbon dioxide can be found in most water bodies like lakes, rivers and oceans. |
Animals that live in the water use the dissolved oxygen in the same way we use the gaseous oxygen found in the air - for cellular respiration
|
| Carbon dioxide is another gas commonly found in water. When it combines with water it forms carbonic acid. This weak acid can slowly dissolve limestone and other rocks. This cave was formed as water slowly dissolved the under ground limestone over hundreds of thousands of years. Some of those dissolved minerals remain in the water. Would you like to know more? |
 |
Exercise 1.2: Make a Naked Egg
Water - Hard and Soft
All natural bodies of water contain dissolved minerals and gasses. Chemicals such as iron, calcium, magnesium and lime can be dissolved in water. When they are found in large amounts, generally greater than 200 mg / litre, it is called hard water.
|
Why is hard water a problem? 1. Lime scale can block hot water heaters, pipes, kettles, dishwashers, shower heads, clothes washers, etc. 2. Scum can build up on bathtubs, sinks, toilets and shower walls. 3. It can leave spots on your dishes and glasses. 4. The water may be discoloured or have a bad taste. 5. Soap does not lather (foam up) as well. 6. Clothing does not feel soft and doesn't get as clean as it should. 7. Hair feels tangled and frizzy. 8. Skin can dry out and conditions like eczema may worsen. |
|
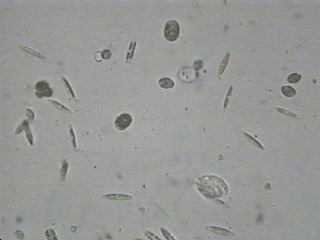
There is much more in a lake, pond or river than dissolved minerals and gasses. As you know from the information you learned in module 2, 'Cells and Systems', most water bodies contain life. For example, algae can be one celled and microscopic but if its present in the water in the billions it will turn the water green in colour. Other forms of life may not be easily seen, but they all have a great impact on the quality of the water. Have you ever wondered where fish go to the bathroom?
|
But You Can't Drink Any Of It - Yet!
 |
One of the great pleasures in life is to hike in the Rocky Mountains. But even here, you shouldn't drink the water in those beautiful mountain streams without treating it first.
 A protozoan parasite called 'Beaver Fever' has contaminated almost all the lakes, rivers and streams in the Rocky Mountain watershed. Symptoms are also described on this website. If you are camping and want to use water from a lake or stream, it's important to ensure that it is clean and safe. Read this website to learn some different methods of purifying water. |
From Fresh Water?
 Many sailors ship wrecked at sea have died of thirst. Do you know why? The water is too salty to drink. Those who do drink it become even more thirsty as their body tries to get rid of the excess salt. SALINITY AND ITS VARIABILITY... Oceanographers report salinity (total salt content) and the concentrations of individual chemical constituents in sea water -- chloride, sodium, or magnesium for example -- in parts per thousand, for which the symbol o/oo is used. That is, a salinity of 35 o/oo means 35 pounds of salt per 1,000 pounds of sea water. Similarly, a sodium concentration of 10 o/oo means 10 pounds of sodium per 1,000 pounds of water. The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, snowfall, wind, wave motion, and ocean currents that cause horizontal and vertical mixing of the saltwater. This page is very detailed (maybe a bit too much!) but it does explain salinity very well. http://www.palomar.edu/oceanography/salty_ocean.htm |
Sea water contains hundreds of dissolved atoms and molecules, including magnesium, calcium, bromine, chlorine, carbonate, sulfur, iron, silicate, and dissolved gases such as oxygen, nitrogen, and carbon dioxide. These, and many more chemicals combine to make the seas and oceans salty. salinity In oceanography, the salinity of seawater is a measure of the amount of total dissolved salts expressed as the number of grams of dissolved material in one kilogram of seawater. It is more strictly defined as the weight in grams of the dissolved inorganic matter in 1 kg of seawater after all bromide and iodide have been replaced by the equivalent amount of chloride, and all carbonate converted to oxide. In practice it is determined from conductivity and temperature measurements in the laboratory or from conductivity, temperature and pressure measurements in situ via the use of a CTD. |