Lesson 5
1. Lesson 5
1.9. Explore 5
Module 5: Rational Expressions

iStockphoto/Thinkstock
The third event at Super Challenge Day is Mixture Madness. Mixing items of two different concentrations can help create the perfect blend to suit your needs, whether you need motor oil and gasoline, trail mix, or ketchup and mustard.
In Mixture Madness, team members combine ingredients to prepare mixtures containing a predetermined proportion of a specified ingredient. Contestants use tools to measure the mass and the volume of the ingredients. Points are deducted according to the discrepancy between the obtained measure and the target measure. The winning team is the team with the fewest deductions.
Try This 4
Can you determine the rational equation that is used to compute the percentage concentration of the solution given a specific volume of water?
Launch the Bleach Solution applet to experiment with different mixtures of concentrated bleach and water. Move the bleach slider to input a volume of bleach that is less than 10 L. Use the water slider to input a volume of water that is less than 100 L. Incremental changes can be made to both sliders using the arrow keys on your keyboard after the sliders are selected. The percentage solution that is created is shown above the container.
Now that you have experimented with the applet, hopefully you have concluded that changing the volume of bleach and/or water changes the percentage concentration of the solution. But can you determine the percentage concentration mathematically?
Consider the following: How much bleach should be added to 50 L of water in order to make a solution that is 10% bleach?
- Remember that percentage concentration is given by
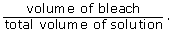 Complete a chart similar to the following to help you observe the patterns involved.
Complete a chart similar to the following to help you observe the patterns involved.
Volume of Bleach (L)
Volume of Water (L)
Percentage Concentration of Bleach
5 L
50 L
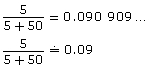
10 L
50 L
15 L
50 L
20 L
50 L
x
50 L
- Using your completed table, write a rational equation that can be used to solve the original problem: How much bleach should be added to 50 L of water in order to make a solution that is 10% bleach? For additional help, use the hints provided.


- Solve the equation. Use the Bleach Solution applet to confirm your answer.
![]() Save your work in your course folder.
Save your work in your course folder.
The expression in the last cell of the third column of the chart is equal to the decimal equivalent of 10%.
The total volume of solution is equal to the volume of bleach plus the volume of water.
