Module 1 Intro
1. Module 1 Intro
1.27. Page 6
Module 1—Energy Flow and the Cycling of Matter
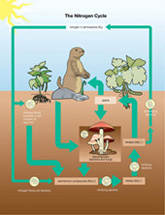
The Nitrogen Cycle
Nitrogen is critically important to life because it is a key component in biologically important molecules, such as protein and DNA. Since the atmosphere is composed of 78% nitrogen, it is reasonable to wonder why you can’t get your nitrogen from breathing. After all, more than three-quarters of every breath is nitrogen!
nitrogen fixation: the process of converting nitrogen gas into ammonia
nitrifying bacteria: a type of soil bacteria that converts ammonia into nitrates and nitrites
nitrification: the process of converting ammonia into nitrates or nitrites
denitrifying bacteria: a type of soil bacteria that converts nitrates in soil into nitrogen gas, releasing this gas to the atmosphere
denitrification: the process of converting nitrates in the soil into nitrogen gas
The trouble with this idea is that nitrogen gas, N2, is non-reactive; it takes a lot of energy to break up N2 molecules so single nitrogen atoms can be combined with other elements to form proteins. Plants and other producers have the same problem—they can’t use atmospheric nitrogen either. The plants rely upon bacteria to convert nitrogen gas, N2, into forms they can use.
These other forms of nitrogen include ammonia (NH3), nitrate ions (NO3-), and nitrite ions (NO2-). These nitrogen-containing substances are found in the waste products of many organisms and in dead and decaying organic matter.
Plants use nitrates or nitrites for their nutrients. For nitrogen gas to be used by plants, the gas has to be converted by certain types of bacteria into ammonia—this is done using a process known as nitrogen fixation.
The bacteria involved in nitrogen fixation are found in the soil and the nodules on the roots of plants called legumes. Other types of bacteria in the soil, known as nitrifying bacteria, convert ammonia into nitrates and nitrites by a process called nitrification.
Since all cycles have to be able to return to the starting point, another soil bacteria known as denitrifying bacteria converts nitrates into nitrogen gas. This process is known as denitrification. Other non-living processes—such as lightning—may convert nitrogen gas into nitrates.
 Read
Read
On this screen, there is text and video that will reinforce what you will be reading in the text. There is quite a bit of vocabulary associated with the nitrogen cycle such as denitrification, nitrogen fixation, and nitrification, so it may be advantageous to make definitions in your own words. You can also make drawings to show what each word means and where the word fits into the nitrogen cycle.
 Self-Check
Self-Check
SC 8. How does the nitrogen cycle diagram, “Figure 2.16,” on page 49 of the textbook compare to the diagram titled “The Nitrogen Cycle.”
 Self-Check Answers
Self-Check Answers
SC 8. Both diagrams show the role of bacteria, plants, and decomposers in the process of cycling nitrogen. The diagram in the textbook shows a terrestrial and aquatic biotic community, outlines that nitrogen is added to water by runoff, and shows that human activities can add nitrates to the cycle. Decomposers are not shown in the biotic community, but they do exist there.
 Watch and Listen
Watch and Listen
View the nitrogen cycle animation by choosing "4.163 Nitrogen Cycle" from the list.
 Read
Read
The phosphorus cycle is much less complex than the nitrogen cycle. What makes it less complex? In your textbook, read page 49, starting at “The Phosphorus Cycle.” Also read pages 50 and 52.
 Self-Check
Self-Check
SC 9. Why do organisms need phosphorus?
SC 10. Describe the cycling of phosphorus.
eutrophication: excessive plant growth and decay caused by an excessive amount of chemical nutrients
SC 11. What happens to a water system when there is an increase in phosphorus?
SC 12. Why could this lead to eutrophication?
 Self-Check Answers
Self-Check Answers
SC 9. Organisms require phosphorus to make ATP, DNA, teeth, and bones. Phosphorus is also part of the cell membrane (i.e., phospholipid bilayer).
SC 10. Phosphorus is transported by water and doesn’t cycle through the atmosphere; phosphorus enters the soil through organisms by waste/decay (rapid cycling) and from the weathering of rock (slow cycling).
SC 11. An algal bloom can occur if there is too much phosphorus in a water system. An excess of nutrients encourages excessive plant growth, which leads to the eutrophication of lakes.
SC 12. Eutrophication would occur because excessive plant growth would block light to other photosynthetic plants, meaning less oxygen produced in the long term. Meanwhile, organisms flourish in the short term due to the increased plant growth but end up using up the available oxygen and contributing carbon dioxide. Also, decaying matter would increase, which would use up oxygen and increase carbon dioxide levels.
 Try This
Try This
TR 2. Draw a unified diagram that illustrates how carbon, oxygen, nitrogen, and phosphorus cycle together through the biosphere.
 Reflect and Connect
Reflect and Connect
As you worked through Lesson 4, the Self-Check questions offered an opportunity to make predictions about what may happen when the biogeochemical cycles are altered in some way.
What would happen if you were missing one of the substances involved in biogeochemical cycles? Would you be able to enjoy your backyard or the outdoors in the same way as you always do? Hopefully, you are over any initial panic about the thought of running out of nitrogen, because you know that nitrogen cycles. Now you should be predicting what effect humans have on these cycles of carbon, oxygen, nitrogen, and phosphorus.
 Reflect on the Big Picture
Reflect on the Big Picture
As seasons cycle, so do nutrients. How do you think seasons affect biogeochemical cycles? How are biogeochemical cycles part of the flow of energy? As you move through this module, focus on how balance and equilibrium are always present in every process that occurs in nature. You also need to think about how you and other people around you can have an effect on this balance.
 Module 1: Lesson 4 Assignment
Module 1: Lesson 4 Assignment
Remember to submit the Assignment answers to your teacher as part of your Module 1: Lesson 4 Assignment.