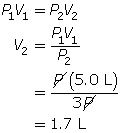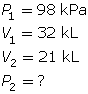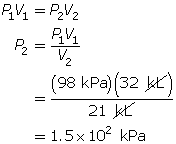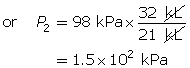Module 3 Intro
1. Module 3 Intro
1.5. Page 3
Module 3—Behaviour of Gases
 Watch and Listen
Watch and Listen
Watch the following video to see an experimental illustration of the effect of pressure on the volume of a gas. You can toggle between the inquiry and explanatory audio tracks as well.
After you have watched the video, summarize what you observed. Where possible, use data from the video to support the points you make. Share your summary with your teacher.
 Read
Read
In the video you observed the effect of exerting a great pressure on the volume of a contained gas. But was there a pattern in changes you observed?
Read “Boyle’s Law” on page 151 in your textbook to learn more about this relationship. Carefully work through COMMUNICATION example 2 on page 151.
 Self-Check
Self-Check
SC 1.
Complete the following table by converting each measurement into the other four units.
kPa |
atm |
mm Hg |
torr |
bar |
|
4.05 |
|
|
|
50.0 |
|
|
|
|
|
|
|
|
1.33 |
|
|
|
770 |
|
|
|
616 |
|
|
SC 2. In Vancouver, where the air pressure is 100 kPa, a child is given a 2.5-L balloon. The family then drives to a higher elevation above sea level, where the air pressure is only 85 kPa. If the temperature is the same in both places, what is the volume of the balloon in the higher location?
SC 3. Complete “Practice” questions 6, 8, and 9 on page 152 in your textbook.
SC 4. A sample of gas is initially at a pressure of 53 kPa and has a volume of 1.8 L. Determine the new pressure if the volume of the gas decreases to 1.2 L.
SC 5. A sample of gas is initially at a pressure of 1.3 atm and has a volume of 2.3 mL. Determine the new volume if the pressure of the gas decreases to 1.1 atm.
SC 6. A sample of gas has a volume of 5.0 L. Determine the new volume of the gas if the pressure is tripled.
 Self-Check Answers
Self-Check Answers
SC 1.
kPa |
atm |
mm Hg |
torr |
bar |
410 |
4.05 |
3.08 x 103 |
3.08 x 103 |
4.10 |
50.0 |
0.493 |
375 |
375 |
0.500 |
133 |
1.31 |
998 |
998 |
1.33 |
103 |
1.01 |
770 |
770 |
1.03 |
82.1 |
0.811 |
616 |
616 |
0.821 |
SC 2. Use Boyle’s law.
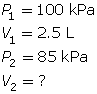
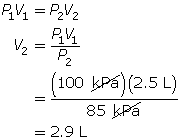
SC 3. Questions 6, 8 and 9 on page 152 of your textbook.
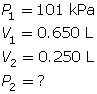
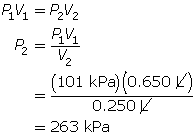
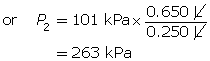
According to Boyle’s law, the final pressure of the air is 263 kPa.
- Pressure and volume are inversely related. If the pressure on the oxygen decreases, the volume should increase if the temperature and the chemical quantity of air remain constant.
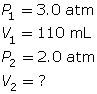
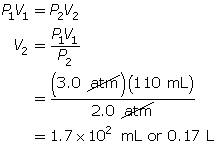
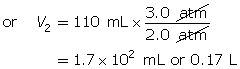
According to Boyle’s law, the final volume of the balloon will be 0.17 L.
SC 4. First, list all the variables.
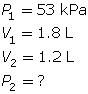
Now, apply Boyle’s law.
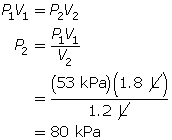
SC 5. First, list all the variables.
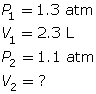
Now, apply Boyle’s law.
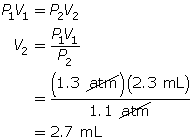
SC 6. Because the pressure is being tripled, call the initial pressure P and the final pressure 3P (3 times the initial pressure).
First, list all the variables.
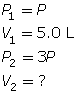
Now, apply Boyle’s law.