Module 3 Intro
1. Module 3 Intro
1.6. Page 4
Module 3—Behaviour of Gases
 Try This
Try This
The Diver—An Application of Boyle’s Law
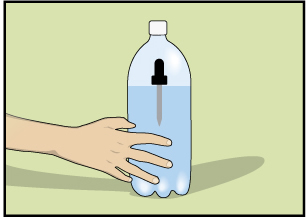
Step 1: Fill a glass with water, draw water into an eyedropper, and place the eyedropper into the glass of water. If it sinks, remove some of the water from the eyedropper. Continue to adjust the water in the eyedropper so that it just barely floats.
Step 2: Fill a clear plastic 1-L or 2-L pop container three-quarters full with water. Carefully place the eyedropper into the pop container without losing any of the water in the eyedropper.
Step 3: Tightly cap the pop container. (The eyedropper should be floating.)
Step 4: Squeeze the sides of the pop container. You should be able to make the eyedropper sink. Releasing the pressure on the sides of the pop container should make the eyedropper float. If this doesn't happen, you will have to adjust the amount of water in the eyedropper.
 Discuss
Discuss
Can you explain why the diver sinks when the pop bottle is compressed? Write an explanation, making explicit reference to Boyle's law and any observations of changes in pressure and volume you observed.
Post your explanation to the discussion area for your class. Read what other students have posted and consider which explanations make the most sense. You may wish to revise your explanation. Save your final version to your course folder.
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
You will complete the following lab as part of your Lesson 1 Assignment. Retrieve your saved copy of the Lesson 1 Assignment now.
 Lab: Boyle’s Law
Lab: Boyle’s Law
The following lab activity will enable you to complete a virtual experiment to investigate Boyle’s law. Ensure you read through the Background, Procedure, and Assignment before beginning the lab.
Complete the questions in Part 1 of the assignment. Save copies of the two graphs you draw for these data.
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
Complete Part 2 of the assignment. Save a copy of your work to your course folder.