Module 3 Intro
1. Module 3 Intro
1.16. Page 3
Module 3—Behaviour of Gases
 Read
Read
The kinetic molecular theory, Boyle’s law, and Charles’ law consider individual or mixtures of unreactive gases. What happens when gaseous substances mix and react? Is there evidence that shows how gaseous substances react with one another?
The Law of Combining Volumes

The law of combining volumes was observed by Joseph Gay-Lussac in 1809. This law states that when temperature and pressure are kept constant, the volumes of gaseous reactants and products in chemical reactions are always in simple ratios of whole numbers. An example is the formation of water, which always requires a ratio of 2 (hydrogen) : 1 (oxygen).
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Volume |
2.0 L |
|
1.0 L |
|
2.0 L |
Volumes of gases were measured because that was empirically possible. But how does a measure of the volumes of gases relate to the number of particles present? Is there a relationship between volume and number of particles in a system?
Avogadro’s Theory
In 1811, Amedeo Avogadro explained Gay-Lussac’s observations. Avogadro noticed the relationship between the ratio of gas volume and the coefficient ratio of a balanced equation.
The coefficient ratio of hydrogen gas to oxygen gas is also 2:1—the same as the gas volume ratio. Finally, there is Avogadro's Theory, which states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. An example is the formation of water.
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Balanced Equation |
2 H2(g) |
+ |
O2(g) |
→ |
2 H2O(g) |
Ratio |
2 |
|
1 |
|
2 |
Volume |
2.0 L |
|
1.0 L |
|
2.0 L |
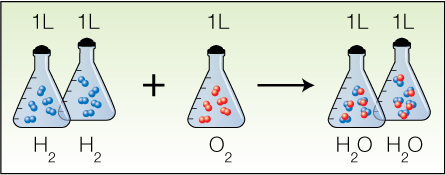
Since equal volumes contain equal numbers of gas molecules, a chemical equation in which all the substances are in the gas phase can be read two ways: as moles of each substance involved, or as litres of each substance involved.
It is important to note that the ratio describes the comparative quantity of each substance in the balanced equation, not the exact quantity. In other words, it does not always have to be 2.0 L of hydrogen, 1.0 L of oxygen, and 2.0 L of water. You must, however, always have twice as much hydrogen as oxygen during the formation of water.
Example
Complete the following table.
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Balanced Equation |
2 H2(g) |
+ |
O2(g) |
→ |
2 H2O(g) |
Ratio |
2 |
|
1 |
|
2 |
Volume |
|
|
225 mL |
|
|
According to the ratio, there is twice as much hydrogen as oxygen. Therefore, the volume of hydrogen must be 2 × 225 mL = 450 mL. Similarly, there is twice as much water as oxygen, which equates to 2 × 225 mL = 450 mL.
For additional clarification of this important law, read “Explaining the Law of Combining Volumes” on pages 164 to 166 in your textbook. Carefully work through “SAMPLE problem 4.2” and the “COMMUNICATION example” in this section.
You may wish to listen to the audio explanation of “SAMPLE problem 4.2.”