Module 4 Intro
1. Module 4 Intro
1.15. Page 4
Module 4—Properties of Solutions
 Watch and Listen
Watch and Listen
In the following videos, you will see how dramatic endothermic and exothermic reactions can be!
Endothermic
Exothermic
 Self-Check
Self-Check
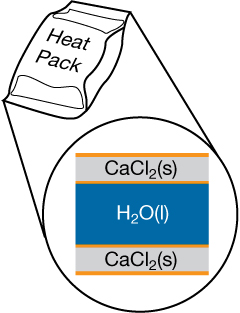
Similar to the cold pack mentioned earlier, a heat pack contains two compartments: a large outer pouch containing calcium chloride, and water stored in the hollow inner cavity. A heat pack is used by breaking the pouch containing the calcium chloride crystals, allowing them to come into contact with water and dissolve.
SC 7. Write the balanced chemical equations for the changes that occur when calcium chloride dissolves in the water in the hot pack.
SC 8. Explain the type of energy changes involved in the hot pack during its operation.
Check your work.
 Self-Check Answers
Self-Check Answers
SC 7. CaCl2(s) → Ca2+(aq) + 2 Cl-(aq)
SC 8. Many energy changes are involved:
- The calcium chloride dissociates, separating the calcium ions from the chloride ions. This is an endothermic process, which absorbs energy.
- The formation of bonds between water and the ions is very exothermic, releasing a lot of energy.
- The overall result is that far more energy comes out of calcium chloride than is absorbed. The overall process is exothermic, and the water heats up.