Module 4 Intro
1. Module 4 Intro
1.40. Page 6
Module 4—Properties of Solutions
 Read
Read
Preparing Solutions
© Linda Muir/shutterstock
Many solutions found in a lab are not actually bought as solutions but as solutes—the solutions are made in the lab by dissolving the solute in water. This procedure greatly reduces the transportation costs of bringing chemical solutions to the end user. A bottle of antacid tablets is economical since you can make 100 antacid solutions from an initial purchase of five dollars. If these 100 solutions all had to be bought separately as solutions, they would cost hundreds of dollars!
Preparing solutions requires the use of specialized glassware. Using appropriate glassware allows for the preparation of solutions with precise concentrations. Here are some of the specialized glassware you will use in preparing solutions.
Volumetric Flask

Volumetric containers are used to measure a specific volume with a very high degree of precision. For example, a 100-mL volumetric flask has a white line that indicates 100 mL. The line is specific for each flask, and its location was determined at standard temperature. This calibration process makes volumetric flasks some of the most precise equipment in a chemistry lab.
Volumetric Pipette
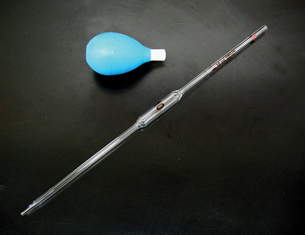
A volumetric pipette is also a calibrated piece of glassware, but it is used for lower volumes. Look at the pipettes in Figures 12 and 13 on the bottom of page 800 of your textbook. A 10-mL volumetric pipette has only one line indicating precisely 10 mL. Graduated pipettes may have markings for each 0.1 mL. See the graduated pipette at the bottom of the page in Figure 13.
 Watch and Listen
Watch and Listen
Preparing a Solution from a Solid
View the video “Preparation of a Solution by Direct Addition.” In this video presentation, you have the opportunity to watch the process to prepare a solution and to answer the questions that accompany the steps in the procedure. Pause the video so you can complete the questions and check your answers. If you experience any difficulty in completing the calculations in the pre-lab, refer to Example 4 below.
In the video the steps involved in making a solution of specific, accurate concentration (a standard solution) are shown. A listing of these steps also appears in "Preparing a Standard Solution from a Solid Reagent" on page 803 of your textbook.
Example 4: A standard solution of 0.350-mol/L iron(II) sulfate is to be prepared. The required volume is 100.00 mL, and the solute has the chemical formula FeSO4 • 7H2O(s). Describe how to prepare this solution.
Step 1: Calculate the mass of solute required.
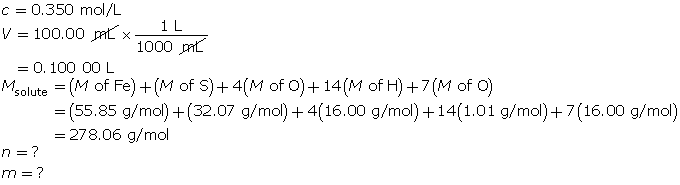
First, determine the number of moles of FeSO4 • 7H2O(s) required.
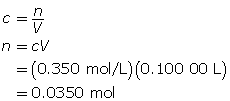
Now, calculate mass of solute required.
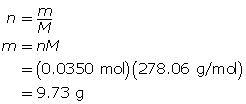
The mass of FeSO4 • 7H2O(s) required is 9.73 g.
Step 2: Measure 9.73 g of iron(II) sulfate heptahydrate in a clean, dry beaker (weighing boat or weighing paper) using an electronic balance.
Step 3: Pour about 50 mL of distilled water into a beaker. Transfer the iron(II) sulfate heptahydrate to the beaker containing the water. Stir to dissolve.
Step 4: Transfer the contents of the beaker into a 100-mL volumetric flask. Rinse the beaker and transfer the rinse water to the volumetric flask.
Step 5: Add distilled water to the volumetric flask until the calibration line is almost reached. Use an eyedropper to add the remaining water so that the bottom of the meniscus meets the calibration line.
Step 6: Stopper the flask and mix the solution by inverting.