Module 5 Intro
1. Module 5 Intro
1.8. Page 6
Module 5—Acids and Bases
 Read
Read
The Hydronium Ion
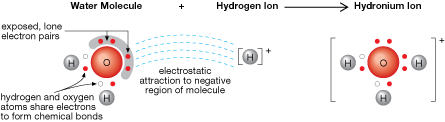
You may recall that the position of lone pairs of electrons around the surface of a water molecule contributes to its polarity. The tendency of these lone pairs is to attract positive charges. Free hydrogen ions, which have a positive charge, are naturally drawn by electrostatic forces toward water molecules, resulting in the formation of hydronium ions.
Examine the structure of the hydronium ion shown in the diagram. Note that all of the hydrogen atoms are covalently bonded to the central oxygen atom. The presence of the lone pair results in a trigonal pyramidal structure for the hydronium ion.
A combination of theoretical and experimental data supports the notion of the hydronium ion as the acidic particle.
- Arrhenius’s theory was unable to explain the mechanism by which an acid “falls apart” (ionizes) to yield the hydrogen ion. The ionization of acids can be explained as a result of collisions between solutes and water molecules, in which a hydrogen ion is transferred to the water molecule, bonding to one of the lone pairs of electrons.
Formation of Hydronium Ions
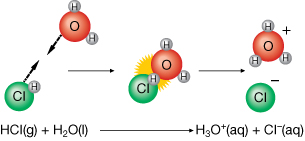
- The observed acidity of substances like NaHSO4(s) can be explained. A reaction between water and dissociated ions can result in the formation of hydronium ions as is summarized in the two reaction equations:
Dissociation of Solute in Water
NaHSO4(s) → Na+(aq) + HSO4–(aq)
Reaction with Water to Form Hydronium Ion
HSO4–(aq) + H2O(l) → H3O+(aq) + SO42–(aq)
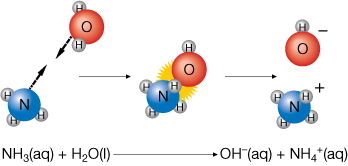
- The observed basicity of ammonia, NH3(aq), can be explained. Ammonia does not possess any hydroxide ions. So how does it form a base? The answer is that ammonia can react with water to produce a hydroxide ion.
Reaction of Ammonia
 Self-Check
Self-Check
Comparing Theories
SC 5. Prepare a table that lists the acidic and basic particles associated with Arrhenius's theory and its more recent revision. Identify the mechanisms by which these particles are formed for each theory.
Test your knowledge of the material covered in this lesson by completing the following questions.
SC 6. State the similarities and differences between a hydrogen ion and a hydronium ion.
SC 7. Write a balanced chemical equation for each situation stated.
- HNO3(aq) reacts with water to form an acidic solution.
- HBr(aq) reacts with water to form an acidic solution.
- Na2HPO4(s) reacts with water to form an acidic solution.
- Na2HPO4(s) reacts with water to form a basic solution.
- Ca(OH)2(s) dissociates to form a basic solution.
- NaCN(s) reacts with water to form a basic solution.
SC 8. Baking soda (sodium hydrogen carbonate) produces a basic solution when dissolved in water. Explain this result using one of the theories you covered in this lesson.
SC 9. Using the modified Arrhenius theory, write the steps in the neutralization reaction between nitrous acid and aqueous sodium hydroxide.
 Self-Check Answers
Self-Check Answers
SC 5.
Theory |
Acidic Particle |
Basic Particle |
Mechanism to Form Particles |
Arrhenius's original theory |
H+(aq) |
OH–(aq) |
|
Arrhenius's modified theory |
H3O+(aq) |
OH–(aq) |
|
SC 6. A hydrogen ion is simply a positively charged proton, whereas a hydronium ion is a water molecule that has incorporated an extra hydrogen atom. A similarity between the two is that they both have a charge of 1+.
SC 7.
- HNO3(aq) + H2O(l) → NO3–(aq) + H3O+(aq)
- HBr(aq) + H2O(l) → Br–(aq) + H3O+(aq)
- Na2HPO4(s) → 2 Na+(aq) + HPO42–(aq)
HPO42–(aq) + H2O(l) → PO43–(aq) + H3O+(aq)
- Na2HPO4(s) → 2 Na+(aq) + HPO42–(aq)
HPO42 –(aq) + H2O(l) → H2PO4–(aq) + OH–(aq)
- Ca(OH)2(s) → Ca2+(aq) + 2 OH–(aq)
- NaCN(s) → Na+(aq) + CN–(aq)
CN–(aq) + H2O(l) → HCN(aq) + OH–(aq)
SC 8. The first reaction involves dissociation of the solid ionic compound.
NaHCO3(s) → Na+(aq) + HCO3–(aq)
The second reaction involves a reaction between the hydrogen carbonate ion and a water molecule.
HCO3–(aq) + H2O(l) → H2CO3(aq) + OH–(aq)
The products of the second reaction, specifically the hydroxide ion, are consistent with the observed basic properties for the solution.
SC 9. HNO2(aq) + H2O(l) → NO2–(aq) + H3O+(aq)
NaOH(aq) → Na+(aq) + OH–(aq)
H3O+(aq) + OH–(aq) → 2 H2O(l)
 Module 5: Lesson 1 Assignment
Module 5: Lesson 1 Assignment
Retrieve the copy of the Module 5: Lesson 1 Assignment that you saved earlier. Complete the questions in Part 3 of your assignment. Save a copy of your answers to your course folder. Once you have completed all of the questions, submit your work to your teacher.