Module 5 Intro
1. Module 5 Intro
1.20. Page 5
Module 5—Acids and Bases
 Read
Read
Strong and Weak Bases
Do strong bases and weak bases exist?
Read “Strong and Weak Bases” on pages 256 and 257 of your textbook. Is the same principle used to differentiate strong and weak acids?
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1 to 5 on page 255 of your textbook.
SC 2. Complete “Practice” questions 6 to 8 on page 257 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 1. “Practice” questions 1 to 5, page 255
- Test 1: If an acidic solution is tested for conductivity and it is highly conductive, then it contains a high concentration of ions [H3O+(aq) and anions] and is a strong acid. If the conductivity is low, then it contains few ions, and is a weak acid.
Test 2: If an acidic solution is tested for pH and it has a low pH, then it contains a high concentration of hydronium ions and is a strong acid. If the pH is high, the solution contains few hydronium ions and is a weak acid.
- The key idea used to explain the difference between strong and weak acids is the extent of the reaction (ionization) of the acid solute with water.
- Examples of strong acids include hydrochloric acid, hydroiodic acid, sulfuric acid, nitric acid, perchloric acid, and hydrobromic acid.
- Hydrochloric acid is a strong acid. Since all strong acids ionize completely (more than 99%), the concentration of hydronium ions produced is 0.15 mol/L.
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq)
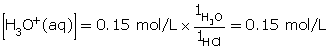
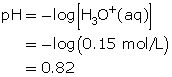
- Hydrochloric acid is a strong acid. Since all strong acids ionize completely (more than 99%), the concentration of hydronium ions produced is 0.15 mol/L.
- Acetic acid is a weak acid and will not ionize completely like hydrochloric acid will. Therefore, a 0.15-mol/L solution of acetic acid will produce a solution containing less than 0.15 mol/L of hydronium ions. Since the concentration of hydronium ions in the acetic acid solution is lower than that of the hydrochloric acid solution, the acetic acid solution should have a pH that is higher than 0.82.
SC 2. “Practice” questions 6 to 8, page 257
- Two possible diagnostic tests and their results are shown below for two basic solutions of equal molar concentration:
Test
Strong Base
Weak Base
conductivity
highly conductive due to complete dissociation and ionization
lower conductivity due to less ionization
pH
very high pH (as expected if nearly all the solute dissociates or ionizes)
lower pH (less than complete ionization)
- Strong bases would have a lower pOH value compared to a weak base with equal concentration of solute (if factors like temperature and concentration were controlled).
- Strong bases are all ionic compounds that contain the hydroxide ion, OH−(aq)
- If you have to explain the basic properties of the substance (presence of hydroxide ion as a product) using a reaction with water rather than by dissociation, the substance is a weak base.
- Strong bases are all ionic compounds that contain the hydroxide ion, OH−(aq)
 Read
Read
Polyprotic Acids and Bases
Earlier in this lesson you learned that phosphoric acid and citric acid are used to flavour soft drinks. Phosphoric acid, H3PO4(aq), has three hydrogen atoms. Each hydrogen is able to react with water, forming a hydronium ion. Citric acid, as shown in the diagram, also contains more than one hydrogen atom that is associated with its acidic behaviour. Acids that have this ability are called polyprotic acids.
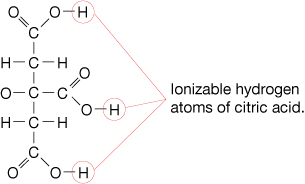
Read “Polyprotic Substances” on page 258 of your textbook for more information about polyprotic acids and polyprotic bases.
 Self-Check
Self-Check
SC 3. Carbonic acid, H2CO3(aq), is a polyprotic acid that is present in soft drinks. Write two balanced chemical equations that involve carbonic acid and the hydrogen carbonate ion, respectively, with water.
SC 4. The phosphate ion, PO43–(aq), can be used in foods to neutralize acids. Use balanced chemical reactions to show the change that can occur within food containing phosphate ions when hydronium ions are also present.
 Self-Check Answers
Self-Check Answers
SC 3.
Reaction 1: H2CO3(aq) + H2O(l) → HCO3–(aq) + H3O+(aq)
Reaction 2: HCO3–(aq) + H2O(l) → CO32–(aq) + H3O+(aq)
SC 4.
Three reactions are possible because phosphate ion is a polyprotic base.
Reaction 1: PO43–(aq) + H3O+(aq) → HPO42–(aq) + H2O(l)
Reaction 2: HPO42–(aq) + H3O+(aq) → H2PO4–(aq) + H2O(l)
Reaction 3: H2PO4–(aq) + H3O+(aq) → H3PO4(aq) + H2O(l)
 Module 5: Lesson 3 Assignment
Module 5: Lesson 3 Assignment
Retrieve the copy of the Module 5: Lesson 3 Assignment that you saved earlier. Complete the questions located in Part 2 of your assignment. Save a copy of your answers to your course folder.
Once you have completed all of the questions, submit your work to your teacher.