Module 7
1. Module 7
1.1. Big Picture
Module 7—Investigating the Nature of the Atom
 Big Picture
Big Picture
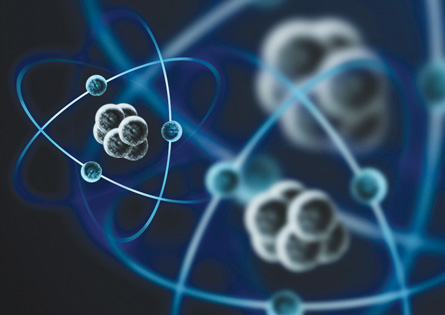
© James Thew/shutterstock
In this artistic representation of the atom, you can see smaller particles orbiting along what appear to be circular paths that enclose a chunky nucleus made up of many smaller particles. Is this really what an atom looks like? Perhaps a better question would be this: Can an atom even be seen? If it could be seen, what would it look like?
Logically, the last question may seem pointless if the answer to the one before it is no.
What is more important, however, is that you understand how the atom is organized so you can predict how it will interact with its environment. And since the atom is too small to see or interact with, a model is used to help bring meaning to the physical reality. Scientists have been working with models of the atom for some time, probing its internal structure and electrical nature. This work has led to the ongoing development of valuable technologies, such as the mass spectrometer, which can identify unknown atoms and elements based on charge and mass.
By the end of Module 7 you will understand how current models of the atom can be applied in technologies used to explore the chemistry of the Earth, the Sun, and other planets in our solar system. You will also see how the practice of science plays out within the social context of the scientific community. You will investigate the nature of the atom.
As you are working in Module 7, keep the following questions in mind:
- How did the cathode ray contribute to the development of atomic models?
- How did J. J. Thomson determine the charge-to-mass ratio of an electron?
- How did Millikan discover the charge of an electron and how did this elementary charge inform models of the atom?
- What did Rutherford’s scattering experiment suggest about the nature of the nucleus? How did it lead to the planetary model of the atom?
- What is the Bohr model of the atom? How are the concepts of stationary states and energy quantization used to explain how a gas absorbs and emits only certain wavelengths of electromagnetic radiation?
Module Assessment
Each lesson has a teacher-marked assignment, based on work completed in the lesson. In addition, you will be graded on your contributions to the Discuss section of each lesson.
You will also be asked to complete Self-Check or Try This questions, which you should place in your Physics 30 course folder. These are not formally assessed, but they are a valuable way to practise the concepts and skills of the lesson. These activities can provide you with reflective feedback on your understanding of the lesson work.
You will be marked for your lesson work on the following items:
- Module 7: Lesson 1 Assignment
- Module 7: Lesson 2 Assignment
- Module 7: Lesson 3 Assignment
At the end of the module you will complete a module assessment that consists of two Diploma Exam-style written-response questions. The first question will assess your knowledge of charge-to-mass ratios and the second question will assess your knowledge of electromagnetic induction. See the Module Summay and Assessment page for more information.