Module 7
1. Module 7
1.4. Page 2
Module 7—Investigating the Nature of the Atom
 Explore
Explore
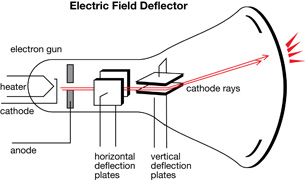
Cathode Rays
All the technology needed to find the electron was available near the end of the 19th century. The manufacturing of sealed glass instruments and tubes had reached a quality that could support low-pressure environments with the operation of a vacuum pump. Around 1850 Heinrich Geissler invented a pump that could produce extremely low pressures. He shaped glass into a tube and evacuated the air from within. Then he filled the tube with a low-density, pure gas. When a potential difference was applied to the electrodes at either end of the tube, a colourful electrical discharge was produced. Further observations indicated that different types of gas emitted a unique and characteristic colour. Geissler called these tubes gas discharge tubes.
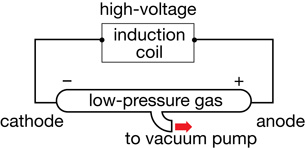
cathode ray: a free electron emitted by a negative electrode in a low-pressure environment
This diagram is an illustration of a simple gas discharge tube.
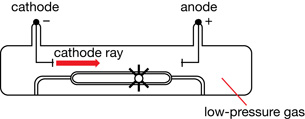
Immediately, the cathode ray became the subject of intense experimentation.
William Crookes proved that the emissions travelled from the cathode to the anode. For this reason, gas discharge tubes are sometimes called Crookes tubes or, more commonly, cathode ray tubes (CRT). Crookes placed a paddle wheel, which was free to rotate, in the path of the cathode rays.
When the rays hit the paddles, they caused the wheel to move along a track inside the tube. This proved that cathode rays were composed of moving particles that had mass and momentum and were capable of doing work.
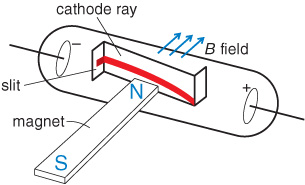
Crookes also demonstrated that cathode rays are deflected by a magnetic field. Using the left-hand rule for moving electric charges in a magnetic field, he proved that the particles were negatively charged and were emitted by the cathode.
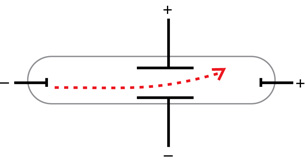
Arthur Schuster used a set of external charged plates to demonstrate that the cathode rays were affected by electric fields. The direction of deflection of the cathode rays further proved that they were, in fact, negative.
Based on these observations, cathode rays
- travel from the negative electrode to the positive electrode
- travel in straight lines and cause shadows
- have momentum and energy to do work
- are deflected by magnetic and electric fields and have a negative charge
 Read
Read
Read “Cathode-ray Experiments” on pages 754 to 755 of your physics textbook. What was the evidence that the cathode rays were particles with charge and mass?
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Remember to submit your answer to A 1 to your teacher as part of your Module 7: Lesson 1 Assignment.
A 1. What was the evidence that the cathode rays were particles with charge and mass?
The Thomson Experiment
In 1897 J. J. Thomson attempted to measure both the mass and charge of the negative particles making up the cathode ray. He was unable to measure either the charge or mass by itself, but he was able to measure them both as a ratio. In other words, he designed an experiment to determine the amount of charge per unit of mass for the particles in the cathode ray. The design of his experiment is based on electric and magnetic forces that act on charged particles. Recall the equations and hand rules that describe these forces.
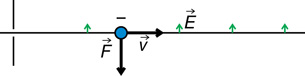
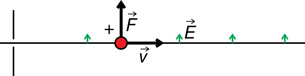
An electrical force can deflect the charged particles in the cathode ray.
![]()
A magnetic force can deflect charged particles in a cathode ray.
![]()

The direction of the force depends on the type of charge and the direction of the electric field. For example,
The direction of the force is determined using hand rules. For example,
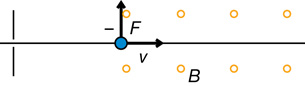
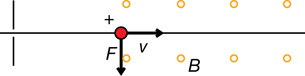
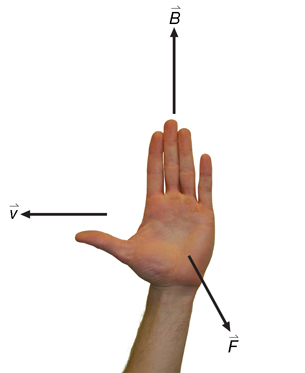
Recall: In the open palm left-hand rule for negative charges, the thumb goes in the direction of movement (![]() ), the fingertips go in the direction of the magnetic field (out of the page in this case), and the palm points in the direction of the force on the charge.
), the fingertips go in the direction of the magnetic field (out of the page in this case), and the palm points in the direction of the force on the charge.
Equation 1
Using only these two forces, Thomson designed an experiment that consisted of two basic investigations. First, he used his apparatus to determine the speed of a cathode ray particle in the tube; then, knowing the speed, he used the apparatus to determine the charge-to-mass ratio of the particle.
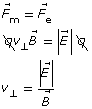
Part 1: Determining the Speed of a Cathode Ray Particle
A magnetic field or an electric field acting alone will deflect a charged particle that is travelling perpendicularly to it.
Thomson used this knowledge to determine the speed of a travelling particle using both a magnetic and electric field simultaneously. He set up the apparatus in such a way that the electric force was oriented opposite to the magnetic force. This way, if the magnetic force were equal in magnitude to the electric force, the particle would travel straight through the apparatus. When the particle travels straight through, Equation 1 can be used to calculate its speed.
 Self-Check
Self-Check
SC 1. A beam of electrons is fired to the right into a set of perpendicular electric and magnetic fields. The electric field is oriented downward and the magnetic field is into the page.
- Explain the direction of the electric force acting on the electrons.
- Explain the direction of the magnetic force acting on the electrons.
- If the electrons travel undeflected, draw a free-body diagram of the electrons.
SC 2. A particle travels undeflected (straight) through a Thomson apparatus that has a magnetic field of 2.0 T and an electric field of 100 V/m oriented perpendicularly to one another. Use Equation 1 to determine how fast it is travelling. Show your work and use the Thomson’s Charge/Mass Measurement simulation to verify your answer by setting the calculated velocity, magnetic field, and electric field as stated. You should observe that the particle travels undeflected through the apparatus if your velocity calculation is correct. Note that the applet will take a few minutes to load.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
- The direction of the electric field is the direction in which a positive particle is pushed; therefore, in a downward field, the negatively charged electron will go the opposite direction, upward.
- Using the left hand rule for negatively charged particles in magnetic fields, the current is travelling to the right (thumb), the magnetic field is into the page (fingers) and the force on the particle is upward (palm faces up).
- Since the electrons are undeflected, the forces—electrical force downward and magnetic force upward—are the same magnitude. The force of gravity is insignificant on such a small particle compared to the other forces, so it is ignored.
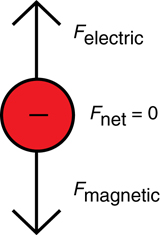
SC 2.
Given
![]()
Required
the velocity of the undeflected particle
Analysis and Solution
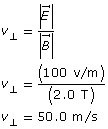
Paraphrase
The velocity of the particle is 50 m/s.