Module 7
1. Module 7
1.6. Page 4
Module 7—Investigating the Nature of the Atom
 Reflect and Connect
Reflect and Connect
One of the most practical uses for Thomson’s ideas is the mass spectrometer like the one used on the Huygens space probe.
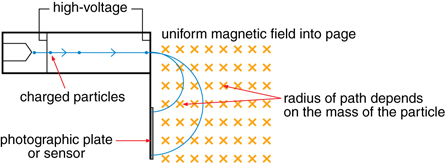
A very large voltage accelerates charged particles. The particles are directed into a magnetic field that is perpendicular to their velocity. Accordingly, a magnetic force is exerted perpendicular to the velocity and the particle travels in a circular path until it hits a photographic plate.
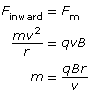
According to the equations that describe a magnetic force that causes circular motion, the radius of the circular path is dependent upon the mass of the particle; therefore, mass can be measured indirectly using the radius of the circular paths.
A mass spectrometer is a powerful application of Thomson’s experiment. In addition to helping humankind explore the chemical composition of other planets and moons, it can be used to identify, by charge and mass, biological chemicals such as toxins, steroids, and drugs. It also has extensive application in pharmaceutical research and quality control in chemical manufacturing.
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Remember to submit your answers to A 6 and RC 1 to your teacher as part of your Module 7: Lesson 1 Assignment.
A 6. A particle accelerated by a potential difference enters a velocity selector. The particle travels straight when the magnetic field is 0.400 T and the electric field is 6.30 × 105 V/m. Once the electric field is turned off, a sensor determines that the radius, or the particle’s path, is 4.11 cm.
- What is the charge-to-mass ratio of this particle?
- Use the charge-to-mass ratio of the particle to determine whether it is an alpha particle, electron, or proton. Hint: Check your physics data sheet for the charges and masses.
RC 1. Complete questions 1 to 3 of “THEN, NOW, AND FUTURE, The Mass Spectrometer” on page 759 of your physics textbook.
Thomson’s Raisin-bun Model
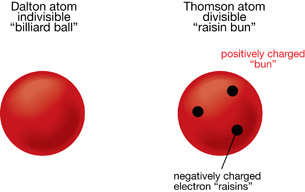
When Thomson started his charge-to-mass ratio experiments, the current atomic model was Dalton’s indivisible billiard ball model. Dalton determined that each element had a unique atom, and from this he inferred that it was impossible to break matter down further. Thomson’s experiment showed that the electron was much smaller than the smallest atom, a hydrogen atom.
Therefore, Thomson determined that an atom was indeed divisible and must consist of a solid, positively charged mass—the bun—with small, negatively charged electrons embedded in it—the raisins. This explained how a cathode ray tube was able to generate a beam of negatively charged cathode rays—electrons—from any metal. This was the first model of the atom that identified positive and negative charges as divisible parts of the atom.
 Self-Check
Self-Check
SC 6. The Dalton model of the atom, developed from chemistry experiments, indicated that the atom was indivisible. Why was J. J. Thomson able to state confidently that electrons were a component of the atom?
SC 7. Why did J. J. Thomson introduce the positively charged “bun” when he didn’t calculate its charge-to-mass ratio?
 Self-Check Answers
Self-Check Answers
SC 6. J. J. Thomson was able to generate cathode rays (electrons) from cathodes made of different elements. This showed that the cathode rays (electrons) came from within many different elements and must be part of the atom that had previously been undiscovered.
SC 7. J. J. Thomson started with neutral atoms (no net charge), which agreed with Dalton’s model. However, the cathode rays coming from the cathode proved to be negatively charged. In order for the atom to be neutral the remaining part of the atom, once the electrons were removed, must be positively charged. The charges of the positive “bun” and negative “raisins” cancelled each other out and resulted in the neutral atom.
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Remember to submit your answer to A 7 to your teacher as part of your Module 7: Lesson 1 Assignment.
A 7. How did Thomson’s discovery of the electron change the current Dalton model of the atom and why was this an extremely significant change?
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Remember to submit the Module 7: Lesson 1 Assignment to your teacher.