Module 7
1. Module 7
1.13. Page 2
Module 7—Investigating the Nature of the Atom
 Explore
Explore


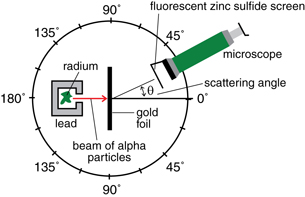
In their experiment, positively charged helium ions (called alpha particles) from a small sample of radioactive radium were used to bombard gold atoms. When the charged alpha particles encountered gold atoms, they were scattered at various angles. The scattered alpha particles could be observed when they encountered a zinc sulfide screen attached to a microscope.
 Read
Read
Read “Rutherford’s Scattering Experiment” on pages 767 and 768 of the textbook.
 Watch and Listen
Watch and Listen
© 2009 University of Colorado. Some rights reserved.
Open the Rutherford Scattering simulation to see how a large nucleus scatters smaller, charged alpha particles. (Note that you will need to save this simulation to your desktop before using it.)
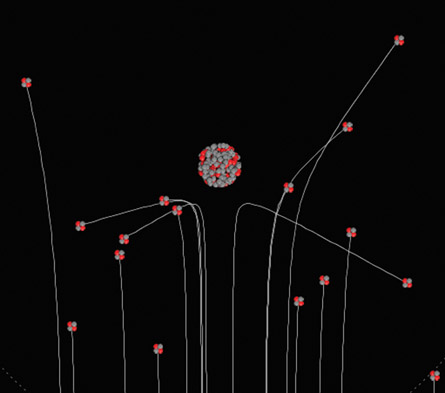
Notice in the simulation that alpha particles are composed of two red protons and two grey neutrons without any electrons, producing the characteristic +2 ion charge. Also notice the small but very distant electron that orbits the large nucleus.
You will see it pass along its circular path in the corners of the viewing area, giving a sense of how small the nucleus and electron are relative to the majority of empty space in the planetary model of the atom.
 Self-Check
Self-Check
SC 1. Adjust the number of protons on the atom using the simulation slider. Set it to 20 protons. Select “Show Traces” to see the path of each alpha particle. Use the term many, few, or rare to complete the following three statements:
- _______ of the alpha particles pass by with little or no scattering, indicating the atom was mostly empty space.
- ______ of the alpha particles are scattered at large angles, indicating the presence of a small, dense nucleus.
- On occasion, _____ alpha particles are scattered straight back toward the source, indicating the presence of a very dense, positively charged nucleus. Presumably, a large electrostatic force of repulsion would be required to reverse the alpha particles’ direction of motion.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
- Many of the alpha particles pass by with little or no scattering, indicating the atom was mostly empty space.
- Few of the alpha particles are scattered at large angles, indicating the presence of a small, dense nucleus.
- On occasion, rare alpha particles are scattered straight back toward the source, indicating the presence of a very dense, positively charged nucleus. Presumably, a large electrostatic force of repulsion would be required to reverse the alpha particles’ direction of motion.
© 2008 University of Colorado. Some rights reserved.
Lab
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Remember to submit your answers to LAB 1 and LAB 2 to your teacher as part of your Module 7: Lesson 3 Assignment.
Open the Rutherford Scattering simulation once again.
LAB 1. Adjust the number of protons to the maximum of 100. Describe what happens to the amount of scattering that occurs and the angles at which it occurs. How can you explain the relationship between the amount of scattering and the number of protons in the nucleus?
LAB 2. Select the “Plum Pudding Atom” from the upper menu on the simulation. Explain why the alpha particles are no longer scattered.

© 2008 University of Colorado. Some rights reserved.
Failure of the Rutherford Model
According to Rutherford, the scattering alpha particles indicated that within the atom there existed a tiny, but very massive, positively charged core. Rutherford concluded that the atom was not filled with a positively charged substance (as Thomson had described); rather, all the positive charge of the atom was located in a nucleus at the centre of the atom. This nucleus was small but contained almost all the mass of the atom. Thus, Rutherford proposed a nuclear model. In this model the atom has a dense nucleus with relatively vast amounts of empty space through which the electrons can pass. The negative charge of the orbiting electrons was the opposite of the positive charge of the nucleus; so, overall, the atom is still electrically neutral, as Dalton determined.
 Watch and Listen
Watch and Listen
Look at the animated Planetary Model of the Atom again. When you follow this link, do not read the lesson to which the link takes you. Instead, scroll down until you see the animated planetary model of the atom.

There was a problem with the nuclear model of the atom. Recall that positive and negative charges attract. If the nucleus were positively charged, then what stopped the electrons from being sucked into the nucleus? To deal with this problem, Rutherford suggested that the electrons orbit the nucleus, much like the moon orbits Earth or like Earth orbits the sun. The force of attraction between the electrons and the nucleus provides the force necessary to keep the electrons in orbit. Hence, Rutherford proposed a planetary model of the atom.
However, the planetary model was also severely flawed. See if you can figure out what the flaw is by answering the following questions.
 Try This
Try This
TR 1. According Maxwell’s theory of electromagnetism, from Module 5: Lesson 1, what happens when an electron is accelerated? What is given off?
TR 2. In Rutherford’s planetary model of the atom, are the electrons accelerated? If so, what force causes the acceleration?
TR 3. What would happen to an atom if electrons emitted radiation as they orbit the nucleus? Would atoms even exist if they constantly lost energy in the form of emitted radiation?
You should have discovered that, according to Rutherford’s model, atoms are not stable and will collapse in on themselves. According to Maxwell’s electromagnetic theory, when charged particles like electrons are accelerated, they emit electromagnetic radiation. Electrons orbiting a nucleus undergo inward acceleration; thus, they should continuously emit electromagnetic radiation. And if the electrons emit electromagnetic radiation, they should be losing energy. And if the electrons lose energy, they will eventually spiral into the nucleus. What was going on? By the end of the 19th century an adequate model of the atom had not yet surfaced.
 Read
Read
Read “The Bohr Model of the Atom” on pages 771 of your physics textbook.
The Role of Atomic Spectra
In developing a model of the atom, scientists also had to contend with atomic spectra. By the early 1800s, scientists knew that every element emits unique line spectra. For example, when an evacuated bulb is filled with neon gas and a voltage is applied to the electrodes, a characteristic red glow is emitted.
Separating this light by wavelength reveals that neon gas is actually emitting a small collection of unique wavelengths that fall in the red to yellow region of the visible light spectrum. You can see these unique wavelengths identified on the line spectrum below the bulbs. If the bulb were filled with a different gas, for example argon, a blue colour would be produced, with a different and unique line spectrum. The line spectrum is like a chemical fingerprint with each element having its own distinct pattern.
Why did each element have unique spectra, and why was it a line spectra? Somehow, these phenomena must be tied into the mystery of atomic structure.
It is important to understand what a line spectrum means. There are three types of spectra: continuous, bright line (emission), and dark line (absorption). A glowing solid or liquid or high-pressure gas will produce a continuous spectrum but only low-pressure gases will produce line spectra.
Continuous Spectra
All wavelengths (and frequencies) of light are present. In the visible range (400 nm to 700 nm), all the colours are visible.

Bright Line Spectra
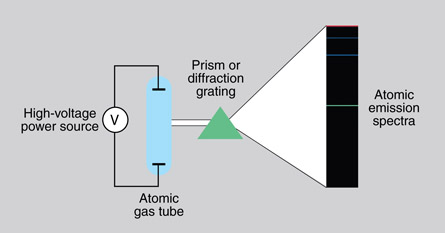
An excited low-pressure gas produces a bright line spectrum. The spectrum only consists of lines of particular wavelength. A bright line spectrum is also called an emission spectrum because the gas emits certain wavelengths (frequencies).

Here is a diagram of the experimental set-up to produce a bright line spectrum.
Dark Line Spectra
A dark line spectrum is created when light from a glowing solid or liquid is passed through an unexcited (cool) gas. The typically continuous spectrum (from the glowing solid or liquid) is now missing certain wavelengths—these missing wavelengths appear as dark lines. A dark line spectrum is called an absorption spectrum because the gas absorbs certain wavelengths (frequencies).

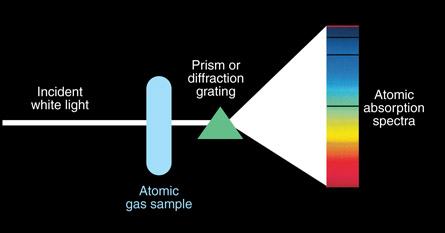
 Self-Check
Self-Check
SC 2. The emission and absorption spectra of hydrogen are displayed above. Compare the position of these lines. What do you notice? Does this mean that a gas can only absorb and emit a limited number of unique EMR wavelengths?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2. Both the emission and absorption spectral lines occur in the same place on the spectrum. This means that a gas can only absorb and emit the same, specific wavelengths of EMR.