Module 2 Intro
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 SS |
| Book: | Module 2 Intro |
| Printed by: | Guest user |
| Date: | Wednesday, 24 December 2025, 12:43 PM |
Description
Created by IMSreader
Table of contents
- 1. Module 2 Intro
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1 Intro
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Page 6
- 1.9. Lesson 2 Intro
- 1.10. Page 2
- 1.11. Page 3
- 1.12. Page 4
- 1.13. Lesson 3 Intro
- 1.14. Page 2
- 1.15. Page 3
- 1.16. Lab
- 1.17. Page 5
- 1.18. Page 6
- 1.19. Lesson 4 Intro
- 1.20. Page 2
- 1.21. Lab
- 1.22. Page 4
- 1.23. Page 5
- 1.24. Page 6
- 1.25. Page 7
- 1.26. Module Summary/Assessment
- 1.27. Module Glossary
1. Module 2 Intro
Module 2—Chemical Compounds
Module Introduction
In Module 2 you will learn more about the bonding of chemical compounds. You may recall that chemical bonding involves attractions between atoms. As you will see in this lesson, forces associated with bonding can be between atoms within a molecule and between molecules. The substances that we use can be considered as chemical technologies—tools to support our lives. In this module you will learn how the physical and chemical properties of many common substances are determined by bonding relationships.
The major concepts developed in this module include
-
bonding theory and Lewis formulas
-
molecular shapes and VSEPR theory
-
molecular polarity
-
intermolecular forces
Think about the following questions as you complete this module:
-
What are the roles of modelling, evidence, and theory in explaining and understanding the structure, bonding, and properties of molecular compounds?
-
Why do substances have different melting and boiling points?
-
How can principles of bonding in matter be used to develop unique materials?
In previous science courses you completed lab activities, submitted reports, and used tables and graphs. These skills will be required in this module. If you require a refresher on any of these areas, review the following pages in your textbook:
-
“Investigation Report Outline” and “Sample Investigation Report,” pages 790 to 795
-
“Laboratory Equipment,” pages 797 to 801
-
“Diagnostic Tests,” page 805
-
“Laboratory Safety,” “Safety Symbols and Information,” and “Waste Disposal,” pages 807 to 810
-
“Tables and Graphs,” pages 815 to 816
1.1. Big Picture
Module 2—Chemical Compounds
 Big Picture
Big Picture
The forces involved in chemical bonding play an integral role in daily life.

© 2007 Jupiterimages Corporation
Settlers to North America used information from local First Nations people, as well as their own intuition, to develop recipes for foods, cleaning products, and weatherproofing that used natural materials (organic and inorganic substances). They found these substances locally or received them by trading with other groups. The substances developed by these people are examples of chemical technologies that have evolved into the commercial products used today.

Rolf Hicker Photography
If you did an inventory of what you ate or the products you used during a day, could you identify the chemical compounds used? How would these compounds be different from the substances used by people living at earlier times in Alberta’s history? In this module you will learn to identify how chemical technologies provide for improved products and how bonding relationships in matter determine the properties of chemical substances and the function of technologies that use them.
1.2. In this Module
Module 2—Chemical Compounds
In This Module
Lesson 1—Bonding Theory and Lewis Formulas
In this lesson you will learn to use electron dot diagrams to represent the arrangement of electrons within an atom and to illustrate how atoms bond together. This lesson will also use the periodic table and Lewis symbols to support and explain ionic bonding theory.
-
What models are used to describe the bonding between atoms in molecular compounds?
-
Can information contained within the periodic table be used to support and explain bonding theories?
Lesson 2—Molecular Shapes and VSEPR Theory
In this lesson you will study the Valence Shell Electron Pair Repulsion (VSEPR) model, recognized as the defining theory for predicting molecular shapes. The molecular shapes you will examine in this lesson are linear, bent, tetrahedral, trigonal pyramidal, and trigonal planar. An introduction to chemical representations of molecules that demonstrate three-dimensional shape will also be included.
-
What is VSEPR theory, and how can it be used to predict molecular shapes?
-
Can the structure of simple molecular substances be illustrated by drawing or building models?
-
How are models and theories useful in helping to explain the structure and behaviour of matter?
Lesson 3—Molecular Polarity
In this lesson you will learn how to determine the polarity of a molecule by analyzing the structural shape and charge distribution within a molecule. Electronegativity, shape, and symmetry all affect the polarity of a molecule. The difference between polar and nonpolar bonds and polar and nonpolar molecules is important in many industrial processes.
-
How can you determine the polarity of a molecule by using simple structural shapes and charge distribution?
Lesson 4—Intermolecular Forces
This lesson focuses on the different types of intermolecular bonding that occur within molecules. Intermolecular bonding includes London forces, dipole-dipole forces, and hydrogen bonding. Intermolecular bonding plays a large role in determining many characteristics of molecular substances, including melting and boiling points, solubility, surface tension, cohesion and adhesion, volatility, and density.
-
What are intermolecular forces?
-
Are the differences between the melting and boiling points of similar substances explained by differences in their intermolecular forces?
- How does scientific knowledge and theory develop through hypothesis, evidence collection, investigation, and explanation?
 Module Assessment
Module Assessment
The assessment for this module consists of four lesson assignments. As you complete these assignments, you will consider some of the larger questions regarding the application of principles of bonding to technologies used throughout history. Your work on these assignments will prepare you to complete the Unit Assessment.
1.3. Lesson 1 Intro
Module 2—Chemical Compounds
Lesson 1—Bonding Theory and Lewis Formulas
 Get Focused
Get Focused

© Loneburro/iStockphoto

© Robert Churchill/iStockphoto
What a difference a century can make, especially when you consider the kind of equipment used to live outdoors. If you compare the two pictures, you can see the influence that chemical technologies have had. In the previous module you learned that many chemical compounds are used to make products. These chemical technologies are often related to the bonding of substances.

Courtesy of The Valley Library, Oregon State University
In the early 1900s, many scientists were asking questions regarding the bonding within chemical compounds. One of the key figures in this discussion was American chemist Gilbert Lewis. In this lesson you will learn about Lewis’ theories and how to represent the arrangement of electrons when atoms bond.
Essential Questions
- What models are used to describe the bonding between atoms in molecular compounds?
- Can information contained within the periodic table be used to support and explain bonding theories?
 Module 2: Lesson 1 Assignment
Module 2: Lesson 1 Assignment
In this lesson you will complete the Module 2: Lesson 1 Assignment. Save a copy of the Lesson 1 Assignment to your course folder. You will receive more information about how to complete the assignment later in this lesson.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check and Try This questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answers to all the questions in this lesson and place those answers in your course folder.
1.4. Page 2
Module 2—Chemical Compounds
 Explore
Explore
 Read
Read
Bonding theory is one of the most important concepts in chemistry. Although you studied bonding theory in Science 10, it is important to review these concepts. Understanding bonding may involve revising what you currently understand. Read the descriptions of different bonding theories in your textbook, on page 78 to the bottom of page 80. List terms that are new to you. You will use this list in the Try This exercise below.
 Try This
Try This
Bonding Concept Map
Prepare a concept map or other graphic organizer that connects any new terms introduced on pages 78 to 80 of your textbook.
To create a concept map, follow these steps:
Step 1: Place terms that you currently use to explain the bonding of matter on a sheet of paper. The order of the terms is not important.
Step 2: Place new terms introduced in the textbook on the sheet of paper.
Step 3: Use lines to connect related terms that appear on the sheet of paper.
Step 4: For each line you draw, write a brief statement (maximum 5 words) that links the two concepts.
Save a copy of your bonding concept map in your course folder. You may wish to share your concept map with your teacher. Later in this module you may wish to review your concept map so that you can add terms, revise linking statements, or add additional lines and linking statements.
 Self-Check
Self-Check
So far in your study of bonding you have seen different representations for electrons associated with atoms. Review the representation for valence electrons shown in “Figure 3” on page 80 of your textbook.
SC 1. Complete the table representing the arrangement of electrons in the following atoms.
a. fluorine
|
e– |
|
|
|
|
|
e– |
|
|||
|
p+(protons) |
||||
|
|||||
F atom |
|||||
b. magnesium
|
e– |
|
|
|
|
|
e– |
|
|||
|
e– |
||||
|
p+ (protons) |
||||
|
|||||
Mg atom |
|||||
 Self-Check Answers
Self-Check Answers
SC 1.
- fluorine
7
e–
2 e–
2 e–
2 e–
1 e–
2
e–
9
p+ (protons)
F atom
- magnesium
2
e–
1 e–
1 e–
0 e–
0 e–
8
e–
2
e–
12
p+ (protons)
Mg atom
 Read
Read
Lewis Symbols
Earlier in this lesson you read about the theories of Gilbert Lewis. In order to support his theory, Lewis developed a model to represent the valence electrons, the electrons associated with bonding.
Read “Atomic Models: Lewis Symbols” on page 81 of your textbook.
 Self-Check
Self-Check
SC 2. Draw a Lewis symbol for sulfur. Identify the lone pairs and the bonding electrons in your diagram.
SC 3. Explain how the location of elements on the periodic table provides information about the number of valence electrons they have.
SC 4. Draw Lewis symbols for all of the group 1 elements. How are these elements similar?
SC 5. Draw Lewis models for all of the group 16 non-metals. How are these elements similar?
 Self-Check Answers
Self-Check Answers
SC 2.

SC 3. The column or group number provides information about the number of valence electrons. Elements in group 1(1A) (first column) all have one valence electron. Information for other columns is listed below.
Group # |
|
Valence |
1 |
IA |
1 |
2 |
IIA |
2 |
13 |
IIIA |
3 |
14 |
IVA |
4 |
15 |
VA |
5 |
16 |
VIA |
6 |
17 |
VIIA |
7 |
18 |
VIIIA |
8 |
SC 4.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
They all have 1 bonding electron.
SC 5.
![]()
![]()
![]()
![]()
They all have 6 valence electrons, 2 bonding electrons, and 2 lone pairs.
 Try This
Try This
TR 1. Lewis symbols, sometimes called electron dot diagrams, are static, two-dimensional representations of an atom. Lewis symbols are a convenient way to show valence electrons. In reality the electrons are in constant motion in three-dimensional space. The organization of orbitals within an energy level can have important considerations.
Use materials available to you at home or school to build three-dimensional representations of Lewis symbols. Use a digital camera or other means to keep a record of your 3-D models in your course folder. Identify what principle is involved in a three-dimensional model to represent the position of valence electrons in orbitals around the atoms. Share your answer to this question with your teacher.
1.5. Page 3
Module 2—Chemical Compounds
Explaining Molecular Formulas Using Lewis Symbols
Since valence electrons are involved in chemical bonding, Lewis symbols should be useful in explaining the changes that occur to atoms when they bond.
Molecular Elements
Seven elements are diatomic, including F2. How could Lewis symbols be used to represent a hydrogen molecule? Explain why this is a diatomic element.
Fluorine has seven valence electrons, requiring one more electron to complete its octet. You recall that atoms tend toward stability, meaning filled orbitals. Can you think of ways that a fluorine atom could complete its one unfilled orbital?
You may recall from your study in the previous module that metals often transfer electrons to non-metals, resulting in the formation of ions that have filled orbitals. Another possible association between atoms with unfilled orbitals is to share electrons. Two fluorine atoms could each obtain a stable octet of electrons if they shared a pair of electrons, forming a covalent bond.

This diagram explains why fluorine is diatomic.
What about oxygen?
double bond: an attraction between atoms in a molecule due to the sharing of two pairs of electrons in a covalent bond
An oxygen atom has six valence electrons. Sharing a pair of bonding electrons like fluorine would leave both oxygen atoms with seven valence electrons, which is less than a stable octet. The solution lies in having a double bond—the two oxygen atoms share two pairs of electrons at once like this:
![]()
structural diagram: a visual representation of a chemical compound that shows the relative placement of every atom within the compound, in addition to all intramolecular bonds
Covalent bonds are represented by the pairs of electrons shown between the Lewis symbols. Covalent bonds can also be represented using a line to represent each shared pair of electrons. The result is a structural diagram. The structural diagram for fluorine (F2) would be written as F–F and for oxygen (O2) as O=O.
 Self-Check
Self-Check
SC 6. Draw the molecular elements chlorine and bromine using Lewis symbols and structural diagrams.
 Self-Check Answers
Self-Check Answers
SC 6.
Chlorine |
Bromine |
|
|
|
|
1.6. Page 4
Module 2—Chemical Compounds
 Read
Read
Molecular Compounds
You have seen how molecular elements are formed through the sharing of electrons. The same principles can be used to explain the bonding of different non-metallic atoms. Read “Molecular Compounds” on pages 86 and 87 of your textbook.
From your reading you should be able to explain how the bonding capacity of an atom can be determined from its Lewis diagram. Can you explain how bonding capacity of the atoms involved in a compound will determine the chemical formula for the compound?
 Try This
Try This
TR 2. Copy into your notebook “Table 1: Bonding Capacities of Some Common Atoms” on page 87 of your textbook. Add rows to your table to provide information for these elements: phosphorous, sulfur, and silicon.
TR 3. Use the information that you just read in the textbook to help you to write steps that describe how to draw Lewis formulas for molecular compounds. Provide an example in your description for a molecular compound. Use examples other than water (H2O) or carbon dioxide (CO2).
 Watch and Listen
Watch and Listen
Watch the animation of the preparation of a Lewis formula for carbon dioxide. Use the animation to check the accuracy of the instructions you wrote.
Example
Draw the Lewis diagram and structural diagram for CH2O, commonly called formaldehyde.
Steps 1 and 2 |
Steps 3 and 4 |
Step 5 |
|
|
|
Submit your answers to TR 2. and TR 3. to your teacher for feedback.
In Chemistry 20 the discussion of molecular bonding is limited to valence electrons and the assumption that all electrons must be paired. In reality, there are compounds that violate these assumptions, but you are not required to study these exceptions in Chemistry 20.
 Self-Check
Self-Check
SC 7. Draw the Lewis formulas and structural diagrams for the following compounds:
hydrogen chloride (HCl)
methane (CH4)
hydrogen peroxide (H2O2)
methanol (CH2O)
 Self-Check Answers
Self-Check Answers
SC 7.
hydrogen chloride
methane


hydrogen peroxide
![]()
methanol

 Watch and Listen
Watch and Listen
Ionic Compounds
Recall from your work in Module 1 that the formation of an ionic compound is a result of the collision between a metal atom and a non-metal atom. This collision results in a transfer of electrons, forming positive and negative ions that have filled energy levels. This transfer is due largely to the difference in electronegativity between the metal and non-metal atoms. The formation of ionic compounds can be represented using Lewis symbols as well. For example, the formation of sodium fluoride can be written as follows:

The formation of an ionic compound, like sodium fluoride, involves a loss of electrons by a metal and a gain of electrons by a non-metal.
The following animation will allow you to see the formation of sodium chloride and magnesium sulfide using Lewis symbols. Notice that magnesium sulfide is MgS rather than Mg2S2. Remember that ionic compounds are referred to by their simplest number ratio.
1.7. Page 5
Module 2—Chemical Compounds
 Reflect and Connect
Reflect and Connect
In this lesson you used Lewis structures and formulas to explain the formation of chemical compounds. Different models of the atom have been used throughout this unit.
Prepare a summary table of the different models of the atom that you have seen so far in this course. For each model, identify what aspects or features of the atom they attempt to represent. Place a copy of your summary in your course folder.
 Reflect on the Big Picture
Reflect on the Big Picture
At the beginning of this lesson you considered how the equipment used for outdoor activities, like camping, has changed over the past century. How have other practices or activities changed as a result of chemical technologies that have been developed?
Talk to an elderly relative or visit a museum in your local area to observe medicines, clothing, tools, foods, or another aspect of human activity. Compare the materials used at that time with those used now. You will likely be amazed at the number of chemicals you encounter.
To keep track of your research, prepare a list that includes the name of the chemical involved, the substance it is found in, the purpose of the product, and the function of the chemical in the product. You may also choose to indicate whether a similar product was used by previous generations, and, if so, what substance (or chemical) was used for that purpose.
Save a copy of your list to your course folder. Later in the module you will be come back to this file and continue to work on it.
 Module 2: Lesson 1 Assignment
Module 2: Lesson 1 Assignment
Retrieve the Module 2: Lesson 1 Assignment that you saved to your computer earlier in this lesson. Complete the questions in the assignment. When you have completed the assignment, save a copy to your course folder and submit a copy of your completed assignment to your teacher.
1.8. Page 6
Module 2—Chemical Compounds
 Lesson Summary
Lesson Summary
In your study in this lesson you considered the following questions:
- What models are used to describe the bonding between atoms in molecular compounds?
- Can information contained within the periodic table be used to support and explain bonding theories?
In this lesson you learned about Lewis structures and formulas. Lewis formulas are commonly used to represent the bonding between atoms in molecular compounds. In the next lesson you will continue to draw Lewis formulas for compounds and further analyze important aspects of the bonding that exists within molecules.
You may wish to prepare answers to the questions listed above as a way to record what you have learned in this lesson. If you do this, place a copy of your answers in your course folder.
Lesson Glossary
double bond: an attraction between atoms in a molecule due to the sharing of two pairs of electrons in a covalent bond
structural diagram: a visual representation of a chemical compound that shows the relative placement of every atom within the compound, in addition to all intramolecular bonds
1.9. Lesson 2 Intro
Module 2—Chemical Compounds
Lesson 2—Molecular Shapes and VSEPR Theory
 Get Focused
Get Focused
© wojciech wojcik/shutterstock
Summer activities are often disturbed by swarms of bloodthirsty mosquitoes. You may include insect repellent on your equipment list when you go camping or enjoy other outdoor activities. In some parts of the world, mosquitoes spread infectious diseases, such as malaria or the West Nile virus. Recently it was discovered that the West Nile virus has reached Alberta, causing the government to launch a public health campaign to minimize exposure to mosquito bites. One prevention method is to use insect repellent, the most common of which contains DEET.
DEET is used now, but what repellent was used by First Nations people and the early settlers in Alberta? You may have heard about alternative insect repellents, including citronella and carbon dioxide. Are these alternatives, or traditional methods, effective? It might surprise you to find that the effectiveness of insect repellent is partially determined by the shape of molecules like DEET.
Essential Questions
-
What is VSEPR theory, and how can it be used to predict molecular shapes?
-
Can the structure of simple molecular substances be illustrated by drawing or building models?
-
How are models and theories useful in helping to explain the structure and behaviour of matter?
 Module 2: Lesson 2 Assignment
Module 2: Lesson 2 Assignment
Save a copy of the Module 2: Lesson 2 Assignment to your course folder. You will receive more instructions about how to complete the assignment later in this lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.10. Page 2
Module 2—Chemical Compounds
 Explore
Explore
 Read
Read
Read page 91 in your textbook. Copy the five statements on VSEPR theory listed at the bottom of the page. Save a copy of these statements in your course folder. You may wish to refer back to this list as you complete the next activity or later when you are reviewing the material from this lesson.
 Try This
Try This
Using VSEPR Theory to Predict Molecular Shape
In this activity you will draw Lewis formulas for chemical compounds and then use the information to consider the three-dimensional shape of these molecules.
Step 1: Print a copy of the handout “Using VSEPR Theory to Predict Molecular Shape.”
Step 2: In the second column on the table, write the Lewis formula for beryllium dihydride, BeH2.
Step 3: Use the Lewis formula drawn in step 2 to complete columns 3 through 5.
central atom: the atom in a molecule that has the most bonding electrons and, therefore, is likely to form the most bonds
Step 4: Use the information from the Lewis formula to determine the distribution of electron pairs in three dimensions around the surface of the central atom, Be. A central atom is the atom in a molecule that has the most bonding electrons and, therefore, is likely to form the most bonds.
Record your answer in column 6 using a term that describes the shape of the molecule around its central atom. You may wish to use a molecular model kit or other materials to represent the atoms involved. If you are using other materials and need assistance, print a copy of the handout “Representing Atoms Using Models” and refer to it as needed.
Step 5: View the video BeH2 to confirm the shape and representation you have determined.
Step 6: Draw the stereochemical formula for BeH2.
Step 7: Repeat steps 2 through 6 for boron trihydride, methane, and ammonia, which are listed in the first column on your table.
Boron trihydride, BH3
Methane, CH4
Ammonia, NH3
Step 8: Read pages 92 and 93 in your textbook. Use the information in the textbook to check the accuracy of your table. If you encounter any difficulties, contact your teacher.
Step 9: Repeat steps 2 through 6 for the remaining substances listed in your table. Use your knowledge of the VSEPR theory to predict the effect that a multiple bond would have on a central atom. To check your work use the information on pages 92 to 95 in your textbook and use the videos listed below for the molecules.
Water, H2O
Hydrogen chloride, HCl
Oxygen, O2
Nitrogen, N2
Carbon dioxide, CO2
 Self-Check
Self-Check
For each compound, draw the Lewis formula and stereochemical formula. Identify its molecular shape as predicted by the VSEPR theory.
SC 1. SF2
SC 2. CCl4
SC 3. PCl3
SC 4. H2S
SC 5. BF3
 Self-Check Answers
Self-Check Answers
SC 1.


angular
SC 2.


tetrahedral
SC 3.


trigonal pyramidal
SC 4.


angular
SC 5.


trigonal planar
1.11. Page 3
Module 2—Chemical Compounds
Reflect and Connect
 Discuss
Discuss
Answer one of the following questions. Post your answers to your class discussion area so that you can share and read ideas from others in your class. Your answer to the second question will consider the influence that shape has on the function of a molecule.
- VSEPR is an abbreviation for valence shell electron pair repulsion. Which word, or words, from the title do you feel are most significant in identifying how this theory can be used to predict molecular shapes? Justify your answer.
Post your answer to your class discussion area. Read the postings from other students. Did most of the students select the same word you did? Were students able to justify their choices by using examples or adequate descriptions?
Before you complete this question, you may wish to take the opportunity to revise your answer by adding detail to the justification you wrote. Place a copy of your completed work in your course folder.

© Lai Leng Yiap/iStockphoto
- Many strategies are used to confuse mosquitoes and prevent their feeding during summer outdoor activities. DEET is a common component of insect repellents. The chemical structure of DEET is shown below.

- Determine the stereochemical shape of the molecule at each of central atoms labelled 1, 2, 3, and 4 in the diagram. If you wish to build a model of the DEET molecule, follow the instructions on the handout “Representing Atoms Using Models” that you printed earlier in the lesson.
- DEET is believed to act by confusing a mosquito’s sense of smell. Evidence shows that DEET blocks the mosquito’s sense receptors that detect the molecule 1-octen-3-ol, which is present in human sweat. If sense receptors rely on molecular shape to be stimulated, comment on the shape of the DEET molecule as compared to 1-octen-3-ol. Suggest how this information could be used to develop other insect repellents.
Submit a copy of your answer to question 2 to your teacher. Use any comments you receive from your teacher to revise your work on this question and on any other questions in this lesson and module.
 Module 2: Lesson 2 Assignment
Module 2: Lesson 2 Assignment
Retrieve the copy of the Module 2: Lesson 2 Assignment that you saved to your course folder earlier in this lesson. Complete all the questions in the assignment.
Save a copy of the completed assignment to your course folder. Submit a second copy of your completed assignment to your teacher.
1.12. Page 4
Module 2—Chemical Compounds
 Lesson Summary
Lesson Summary
In this lesson you investigated the following essential questions:
-
What is VSEPR theory, and how can it be used to predict molecular shapes?
-
Can the structure of simple molecular substances be illustrated by drawing or building models?
-
How are models and theories useful in helping to explain the structure and behaviour of matter?
In this lesson you studied the VSEPR theory and used it to construct, illustrate, and predict the shape of molecular substances. You have also used the shapes predicted using the VSEPR theory to explain the structure and behaviour of matter.
Lesson Glossary
central atom: the atom in a molecule that has the most bonding electrons and, therefore, is likely to form the most bonds
1.13. Lesson 3 Intro
Module 2—Chemical Compounds
Lesson 3—Molecular Polarity
 Get Focused
Get Focused

© Paul Hart/iStockphoto
Mmmm, pancakes. Although pancakes are great to eat, sometimes cooking them can be tricky. Many people like to use non-stick cookware to prevent pancakes and other foods from sticking to the metal surface of their cookware. What is special about the coating that prevents food from sticking? Does this property relate to an aspect of the bonding of the molecules used to make the coating?
Essential Question
-
How can you determine the polarity of a molecule by using the shape of its structure and its distribution of charge?
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
Save a copy of the Module 2: Lesson 3 Assignment to your course folder. You will receive more information about on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.14. Page 2
Module 2—Chemical Compounds
 Explore
Explore
In previous science courses you learned about the unique properties of water, including its high surface tension and high boiling point. You may recall hearing that these properties were due to the polarity of the water molecule. How can you test to see if a molecule is polar? Complete the next investigation to find out.
 Try This: Bending a Stream of Water
Try This: Bending a Stream of Water
Can you cause a thin stream of water from a tap to bend without actually touching the water?
Materials
- a plastic object (e.g., a bendable plastic ruler, plastic ballpoint stick pen, or comb) or a balloon. The balloon works best, especially when you rub it on your hair.
- a piece of cotton (e.g., a tea towel or cotton shirt) or a piece of fur
Procedure
Step 1: Rub the plastic object with the cotton or fur or rub the balloon in your hair. Make sure you rub vigorously to build up a good static charge on the object.
Step 2: Turn on the tap to allow a thin stream of water to pour out. Kitchen taps work well. The thinner the stream, the more observable the change will be.
Step 3: Bring the plastic object close to, but do not touch, the stream of water. What do you notice? Record your observations.
Step 4: Record your observations when you approach the stream of water with the plastic object from the opposite side.
Step 5: If you have a piece of wool handy, try rubbing it on the plastic object. This will place an opposite charge on the object. Repeat Steps 3 and 4. Record your observations.
Save a copy of your data to your course folder. You may wish to refer to your investigation and your data later in this lesson. Send a second copy of your data to your teacher.
1.15. Page 3
Module 2—Chemical Compounds
 Read
Read
In Lesson 3 of Module 1 you were introduced to electronegativity, the attraction that an atom has for a shared pair of electrons. In that lesson you calculated differences in electronegativity between atoms; and when the difference was large, you classified these bonds as being polar.
You may wish to reread the section titled “Electronegativity and Bond Polarity” on pages 99 and 100 in your textbook.
Your observation of the bending of the stream of water when exposed to a charged object, demonstrates the polarity of the water molecule. Polarity means having two different regions of charge.
bond dipole: the charge separation that occurs when the electronegativity difference of two bonded atoms shifts the shared electrons, making one end of the bond partially positive and the other partially negative
As you have seen, it is possible to have a bond dipole. A bond dipole is the charge separation that occurs when the electronegativity difference of two bonded atoms shifts the shared electrons, making one end of the bond partially positive and the other partially negative.
What happens when a molecule is composed of many atoms?
Read the section, “Bond Polarity and Molecular Polarity” on pages 101 and 102 in your textbook. Work through “SAMPLE problem 3.5” on page 102 in your textbook.
 Try This
Try This
TR 1. Explain why the stereochemistry (shape) of a molecule needs to be considered when determining if a substance is polar. To support your answer, use one example other than the ones discussed in your textbook. Save a copy of your answer to this question to your course folder. Send a second copy to your teacher.
Water is a polar molecule. This means that although water molecules are neutral, the electron charge within the molecule is not symmetrically distributed. The oxygen portion of the water molecule is slightly negative, while the hydrogen portions are slightly positive. Because there is a difference of charge within the molecule, there is a negative pole and a positive pole.

Notice that the overall charge of the molecule is zero, although the side with the hydrogen atoms is slightly positive and the side with the oxygen atom is slightly negative.
The molecules in liquid form are loosely packed together and are able to rotate. If a negatively charged object is brought close to the water stream, the molecules within the water will orient themselves so that the slightly positive hydrogen atoms will be attracted to the negatively charged object.
Conversely, if a positively charged object is brought close to the stream of water molecules, the molecules will orient themselves so that the slightly negative oxygen atoms are attracted to the positively charged object.

This action leads to the water being attracted to both types of charges; therefore, the water always bends toward the object. A bond dipole is the charge separation that occurs when the electronegativity difference of two bonded atoms shifts the shared electrons, making one end of the bond partially positive and the other partially negative.
nonpolar molecule: a molecule in which the negative (electron) charge is distributed symmetrically among the atoms making up the molecule
A nonpolar molecule has a symmetrical electron distribution—there is no positive pole or negative pole. Diatomic elements, such as fluorine, are nonpolar.
 Self-Check
Self-Check
SC 1. Complete question 14 at the top of page 103 in your textbook.
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
You will use the following lab to complete part of your Module 2: Lesson 3 Assignment.
1.16. Lab
Module 2—Chemical Compounds
 Lab: Evidence for Polar Molecules
Lab: Evidence for Polar Molecules
In this lesson you learned how to predict if a substance is polar, but are your predictions verified by experiment?
Problem
Which of the liquids have polar molecules?
Retrieve your Module 2: Lesson 3 Assignment that you saved to your course folder earlier in this lesson.
-
Complete Part 1: Pre-Lab in the Lesson 3 Assignment.
-
Complete the virtual investigation of the procedure described on page 131 of your textbook. Use a table to record your data in Part 2: Data in your Lesson 3 Assignment.
-
Complete Part 3: Analysis of Data in your Lesson 3 Assignment.
Save a copy of your work to your course folder.
1.17. Page 5
Module 2—Chemical Compounds
 Reflect and Connect
Reflect and Connect
The molecule used to make the non-stick coating in many types of cookware is shown below. Determine the polarity of this molecule. How does its polarity compare with water?

You may recall that oil and water do not mix. Oils are nonpolar substances, and as you will learn, polarity is an important consideration if you want substances to mix or to go into solution. In the case of cookware, a nonpolar surface provided by the non-stick coating repels water and most of the food particles, preventing their sticking.
Can you predict what might happen to the performance of non-stick cookware if the coating on the surface is scratched?
 Reflect on the Big Picture
Reflect on the Big Picture
Knowledge of polar and nonpolar substances is essential in many industries. In the cleaning business, the removal of grease, oil, dirt, and other stains can be facilitated by using a compound with the “correct” polarity.
You may have seen stainfree and wrinkle-free fabrics. Take a look at the clothes that you wear—how many are made from special materials that prevent stains, are wrinkle resistant, are designed to stay dry (remove perspiration), and repel water?
Make a list of the fabrics that are used. Consider whether similar types of materials existed 100 years ago and, if so, what was used?
Briefly explain how polarity is involved in the function of current and past fabrics that were intended to have a function. Place a copy of your answers in your course folder.
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
Retrieve the copy of the Module 2: Lesson 3 Assignment to which you have saved your previous work. Complete question 6. Save your work to your course folder.
After you have completed all parts of the assignment, submit a copy of your Module 2: Lesson 3 Assignment to your teacher.
1.18. Page 6
Module 2—Chemical Compounds
 Lesson Summary
Lesson Summary
In this lesson you investigated the following essential question:
-
How can you determine the polarity of a molecule by using simple structural shapes and charge distribution?
As part of your study you learned to use electronegativities to determine both bond dipoles, and then to consider the shape of molecules to determine their polarity. You predicted the polarity of different substances and performed an experiment to test your predictions.
Lesson Glossary
bond dipole: the charge separation that occurs when the electronegativity difference of two bonded atoms shifts the shared electrons, making one end of the bond partially positive and the other partially negative
nonpolar molecule: a molecule in which the negative (electron) charge is distributed symmetrically among the atoms making up the molecule
polar molecule: a molecule in which the negative charge is not distributed symmetrically among the atoms, resulting in partial positive and negative charges on opposite ends of the molecule
1.19. Lesson 4 Intro
Module 2—Chemical Compounds
Lesson 4—Intermolecular Forces
 Get Focused
Get Focused

Although we cannot see intermolecular forces, they do influence the behaviour of matter. In the previous lesson you observed how the polarity of a molecule can be examined, and you learned that the polarity of compounds is important to their function in a technology like non-stick cookware. It might not seem immediately apparent when your car overheats on a deserted stretch of road, but boiling point is another property of matter that is influenced by intermolecular bonds. You may recall from your driver training course that antifreeze is an important substance that helps to maintain a car’s performance. How does antifreeze work?
In this lesson you will learn about different types of intermolecular bonding forces and how they are believed to influence the properties of matter.
Essential Questions
-
What are intermolecular forces?
-
Are the differences between the melting and boiling points of similar substances explained by differences in their intermolecular forces?
-
How does scientific knowledge and theory develop through hypothesis, evidence collection, investigation, and explanation?
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Save a copy of the Module 2: Lesson 4 Assignment to your course folder. You will receive more information about how to complete the assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.20. Page 2
Module 2—Chemical Compounds
 Explore
Explore
 Read
Read
intermolecular force: attraction and repulsion between molecules
intramolecular force: attraction and repulsion within a molecule; typically covalent bonds
momentary dipole: an uneven distribution of electrons around a molecule, resulting in a
temporary charge difference between its ends
Your study in Module 1 focused on reviewing intramolecular forces. In this module you have been learning about intermolecular forces.
Read about intermolecular forces on pages 105 and 106 in your textbook.
 Watch and Listen
Watch and Listen
View the animation that illustrates the formation of a momentary dipole between carbon atoms. A momentary dipole is an uneven distribution of electrons around a molecule, resulting in a temporary charge difference between its ends. The predicted existence of momentary dipoles is an important part of the hypothesis supporting the London force. How is it possible for atoms to have momentary dipoles?
View the animation that illustrates the attractions between polar molecules. Can you recall what causes molecules to be polar?
Is there a similarity between London forces and dipole-dipole forces?
Make sure you answer these questions as you complete the next activity.
 Try This
Try This
Construct a table to summarize the differences between intramolecular and intermolecular bonding forces.
In your table provide examples of each type of bonding and a brief description. For each example, indicate factors that will influence the strength of the respective type of bond.
Send a copy of your table to your teacher for feedback.
1.21. Lab
Module 2—Chemical Compounds
 Lab: Boiling Points of Hydrocarbons
Lab: Boiling Points of Hydrocarbons
Background Information
When a substance boils, molecules leave the liquid phase and enter the gaseous phase. In order to make this transition to a higher energy phase, they must have sufficient energy to overcome attractive forces between neighbouring molecules in the liquid phase. It is hypothesized that molecules with greater intermolecular forces have higher boiling temperatures.
Purpose
The purpose of this investigation is to test the hypothesis relating boiling temperature to strength of intermolecular forces.
Problem
Do molecules with larger intermolecular forces have higher boiling temperatures?
Procedure
Step 1: Open the learning object, Boiling Points of Hydrocarbons.
Step 2: Follow the instructions shown in the learning object. Record your observations in the form of a table.
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Retrieve Module 2: Lesson 4 Assignment that you previously saved to your course folder.
Complete question 1, Lab: Boiling Points of Hydrocarbons, in the Lesson 4 Assignment.
1.22. Page 4
Module 2—Chemical Compounds
 Read
Read
Read “Using Dipole-Dipole and London Forces to Predict Boiling Points” on pages 107 and 108 in your textbook. After reading this section, answer the questions below. Send your answers to your teacher.
-
Does the data shown in "Table 1" on page 107 support the hypothesis relating number of electrons and boiling point? Explain your reasoning.
-
State the name of the type of intermolecular bonding force that is influenced by number of electrons.
-
Was the comparison between hydrocarbons in the lab, Boiling Points of Hydrocarbons, a valid set of compounds to examine to study this type of relationship? Support your answer by making specific reference to the structure and polarity of the compounds involved.
 Self-Check
Self-Check
SC 1. Complete "Practice" problem 1 on page 109 of your textbook.
 Self-Check Answer
Self-Check Answer
SC 1. a. London, dipole-dipole (Water is a polar substance.)
b. London
c. London
d. London, dipole-dipole (Ethanol is a polar substance.)
e. London, dipole-dipole (Ammonia is a polar substance.)
f. London
SC 2. Complete "Practice" problem 2 on page 109 of your textbook.
 Self-Check Answer
Self-Check Answer
SC 2.
- hydrogen fluoride. The difference in electronegativity between H and F is greater than the difference between H and Cl, resulting in stronger bond dipoles.
- CH3Cl. The bond dipole between C and Cl is greater than C and I.
- Ammonia, NH3. The bond dipole between N and Hl is greater than N and Br.
- Water. The bond dipole between H and O is greater than H and S.
SC 3. Complete "Practice" problem 3 on page 109 of your textbook.
 Self-Check Answer
Self-Check Answer
SC 3.
Compound
Number of Electrons
methane
10
ethane
18
Ethane will have stronger London forces since it has the greater number of electrons.
Compound
Number of Electrons
oxygen
12
nitrogen
14
Nitrogen will have stronger London forces since it has the greater number of electrons.
Compound
Number of Electrons
sulfur dioxide
32
nitrogen dioxide
23
Sulfur dioxide will have stronger London forces since it has the greater number of electrons.
Compound
Number of Electrons
methane
10
ammonia
10
The two compounds being compared are isoelectronic; therefore, if the London force is the only intermolecular bonding force, then they should have the same boiling point.
SC 4. Complete "Practice" problem 4 on page 109 of your textbook.
 Self-Check Answer
Self-Check Answer
SC 4.
Compound
Number of Electrons
Stereochemical Shape
Polar
boron trifluoride
32
trigonal planar
no
nitrogen trifluoride
34
trigonal pyramidal
yes
Nitrogen trifluoride would have the higher boiling point since it has the larger number of electrons; therefore, has stronger London forces, is polar, and will have additional dipole-dipole forces.
Boron trifluoride is nonpolar, has only London forces, and has fewer electrons; therefore, it has relatively weaker attractive forces.
Compound
Number of Electrons
Stereochemical Shape
Polar
chloromethane, CH3Cl
26
tetrahedral
yes
ethane, C2H6
18
tetrahedral
nonpolar
Chloromethane would have the higher boiling point of the two compounds but stronger London forces and dipole-dipole forces due to its polarity.
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Retrieve the copy of the Module 2: Lesson 4 Assignment that you saved to your course folder earlier.
Complete questions 2.a. and b. of the assignment.
1.23. Page 5
Module 2—Chemical Compounds
 Read
Read
Can you think of a reason why the boiling points of some compounds cannot be explained using the hypothesis for London and dipole-dipole forces? Maybe another type of intermolecular bonding force exists.
Read “Hydrogen Bonding” on pages 111 and 112 in your textbook.

 Watch and Listen
Watch and Listen
View the animation of the hydrogen bonding in ammonia.
Use a diagram of your choice to explain why hydrogen atoms become attracted to neighbouring nitrogen atoms in ammonia. Send a copy of your diagram to your teacher.
The boiling points of water, ammonia, and hydrogen fluoride demonstrate that the hydrogen bonding is stronger than other dipole-dipole forces.
 Self-Check
Self-Check
SC 5. Complete the following table. The first row has been done for you.
Molecule |
Structural Formula |
Shape |
Polarity |
Types of Intermolecular Forces |
HBr |
H–Br |
linear |
polar |
dipole-dipole |
HF |
|
|
|
|
AsH3 |
|
|
|
|
BF3 |
|
|
|
|
HI |
|
|
|
|
 Self-Check Answer
Self-Check Answer
SC 5.
Molecule |
Structural Formula |
Shape |
Polarity |
Types of Intermolecular Forces |
HBr |
H–Br |
linear |
polar |
dipole-dipole |
HF |
H–F |
linear |
polar |
hydrogen bonding |
AsH3 |
H – As – H |
trigonal pyramidal |
polar |
dipole-dipole |
BF3 |
F – B – F |
trigonal planar |
nonpolar |
London |
HI |
H–I |
linear |
polar |
dipole-dipole |
 Read
Read
Read “Physical Properties of Liquids” on page 113 of your textbook.
1.24. Page 6
Module 2—Chemical Compounds
 Reflect and Connect
Reflect and Connect
At the beginning of this lesson, you considered the function of antifreeze in an automobile engine. Engine coolants often contain alcohol compounds like ethane-1,2-diol, pictured here.
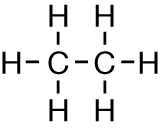
An antifreeze, or engine coolant, is responsible for transferring heat from the engine by circulating it through the radiator. Antifreeze is a solution containing mainly water and the coolant molecule. The boiling point of antifreeze is much higher than pure water. Knowing what you do about chemical structures and intermolecular bonds, can you suggest a reason?
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Place your answer to the question posed above in the Reflect and Connect section into question 3 of the Lesson 4 Assignment. Save your assignment to your course folder.
 Going Beyond
Going Beyond
You may be interested in cloud seeding—a process that takes place in Alberta, particularly in the corridor between Red Deer and Calgary. For more information read “Web Quest—Cloud Seeding” on page 112 of your textbook.
You may also be interested in the case study “Current Research on Intermolecular Forces” on pages 114 and 115 of your textbook. This case study examines the work of Canadian scientist Dr. Robert J. Le Roy.
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Retrieve the copy of the Module 2: Lesson 4 Assignment that you saved to your course folder.
Complete questions 4, 5, and 6 of the Lesson 4 Assignment. Save your work to your course folder. Send a copy of the completed Lesson 4 Assignment to your teacher.
1.25. Page 7
Module 2—Chemical Compounds
 Lesson Summary
Lesson Summary
In this lesson you focused on the following essential questions:
-
What are intermolecular forces?
-
Are the differences between the melting and boiling points of similar substances explained by differences in their intermolecular forces?
-
How does scientific knowledge and theory develop through hypothesis, evidence collection, investigation, and explanation?
Throughout your work in this lesson, you focused on boiling points and empirical data, and you developed a conceptual understanding of how intermolecular bonding forces could be responsible for the phenomena observed.
The melting and boiling points of a substance are related to the strength of intermolecular forces between the molecules of that substance. Higher melting and boiling points indicate stronger intermolecular forces. With an increase in the overall size of the molecule, there is an associated increase in the number of electrons. This increase, too, will increase the strength of intermolecular bonds and lead to higher melting points and boiling points.
You also examined some of the physical properties of liquids, focusing on solubility, surface tension, cohesion and adhesion, volatility, and density and how these could be explained by the intermolecular forces you learned about in this lesson.
Lesson Glossary
intermolecular force: the relatively weak forces of attraction and repulsion between molecules
intramolecular force: the relatively strong bonds or forces of attraction and repulsion within a molecule; typically covalent bonds
momentary dipole: an uneven distribution of electrons around a molecule, resulting in a temporary charge difference between its ends
1.26. Module Summary/Assessment
Module 2—Chemical Compounds
 Module Summary
Module Summary
As you worked through this module, you kept the following questions in mind. Did your studies help you answer these questions?
- What are the roles of modelling, evidence, and theory in explaining and understanding the structure, bonding, and properties of molecular compounds?
- Why do substances have different melting and boiling points?
- How can principles of bonding in matter be used to develop unique materials?
Throughout your study in this module you learned about many technologies involving molecular compounds and about how the bonding of these compounds can be used to make materials useful to you. Chemical principles you studied include electronegativity, bond polarity, VSEPR theory, stereochemistry, molecular polarity, and different types of intermolecular bonding forces including London, dipole-dipole, and hydrogen bonds.
 Module Assessment
Module Assessment
The assessment for this module consists of four lesson assignments. As you completed these assignments, you have also considered some of the larger questions regarding the application of principles of bonding to technologies used throughout history. Your work on these assignments will prepare you to complete the Unit Assessment exercise.
1.27. Module Glossary
Module 2—Chemical Compounds
Module Glossary
bond dipole: the charge separation that occurs when the electronegativity difference of two bonded atoms shifts the shared electrons, making one end of the bond partially positive and the other partially negative
central atom: the atom in a molecule that has the most bonding electrons and, therefore, is likely to form the most bonds
double bond: an attraction between atoms in a molecule due to the sharing of two pairs of electrons in a covalent bond
intermolecular force: the relatively weak forces of attraction and repulsion between molecules
intramolecular force: the relatively strong bonds or forces of attraction and repulsion within a molecule; typically covalent bonds
momentary dipole: an uneven distribution of electrons around a molecule, resulting in a temporary charge difference between its ends
nonpolar molecule: a molecule in which the negative (electron) charge is distributed symmetrically among the atoms making up the molecule
polar molecule: a molecule in which the negative charge is not distributed symmetrically among the atoms, resulting in partial positive and negative charges on opposite ends of the molecule
structural diagram: a visual representation of a chemical compound that shows the relative placement of every atom within the compound, in addition to all intramolecular bonds


