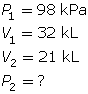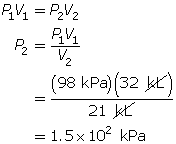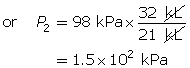Module 3 Intro
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 SS |
| Book: | Module 3 Intro |
| Printed by: | Guest user |
| Date: | Wednesday, 24 December 2025, 10:32 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 3 Intro
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1 Intro
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Page 6
- 1.9. Lesson 2 Intro
- 1.10. Page 2
- 1.11. Lab
- 1.12. Page 4
- 1.13. Page 5
- 1.14. Lesson 3 Intro
- 1.15. Page 2
- 1.16. Page 3
- 1.17. Page 4
- 1.18. Page 5
- 1.19. Page 6
- 1.20. Lesson 4 Intro
- 1.21. Page 2
- 1.22. Page 3
- 1.23. Page 4
- 1.24. Lab
- 1.25. Page 6
- 1.26. Page 7
- 1.27. Module Summary/Assessment
- 1.28. Module Glossary
1. Module 3 Intro
Module 3—Behaviour of Gases
Module Introduction
Throughout Module 3, models of the gaseous state of matter will be used to explain molecular behaviour. The major concepts developed in this unit are
- properties of gases and Boyle’s law
- Kelvin temperature, Charles’ law, and the combined gas law
- law of combining volumes and Avogadro’s theory
- molar volume and the ideal gas law
Think about the following questions as you complete this module:
- What is the relationship among pressure, temperature, volume, and amount of a gas?
- How is the behaviour of gases used in various technologies?
Module 3 will culminate with a final assignment. For more information on the assignment and how you will be assessed, refer to the Unit Assessment section.
1.1. Big Picture
Module 3—Behaviour of Gases
 Big Picture
Big Picture
The term gas was first used in 1663 to describe a specific form of matter. Johann von Helmont, a Belgian physician, derived the word from the Greek word chaos, which referred to the “great abyss” from which Earth was supposedly formed. Some have suggested that von Helmont chose the word chaos because his equipment kept exploding when he tried to make gases.
Gases have many easily observable properties that have made them very interesting to study. Gases are fluids, meaning they fill whatever space is available, and they can be compressed by applied pressure. They are affected by temperature, can flow readily from place to place, and have mass.
Watch the following video. You may be required to login with a username and password. Contact your teacher for this information. As you watch the video, select the inquiry track. Write down your answers to the questions that are asked on the video. To check your answers, you can review this video with the explanatory track selected.
http://www.learnalberta.ca/content/secr/Gases/expanding_marshmallow/chem.html
Read through the Case Study “Compressed Gases” on pages 159 and 160 of your textbook to gain an appreciation of some of the ways compressed gases are used.
In this module you will investigate the empirical relationships that exist among pressure, volume, temperature, and amount of gas in a system. You will also use your understanding of bonding concepts from Unit A to explain at a theoretical level the behaviour you observe.
The following questions will be investigated in this module:
- What is pressure?
- How is pressure measured?
- How are pressure and volume of a gas related?
- How are pressure-volume relationships applied in technologies to support breathing?
- What is the Kelvin temperature scale, and why is it used?
- What are the relationships among amount, pressure, volume, and temperature for a fixed amount of gas?
- Is it possible to predict the quantity of a gaseous substance involved in a chemical reaction?
- What is molar volume?
- What is the difference between a real gas and an ideal gas?
- What relationship exists between pressure, volume, temperature, and moles of gas?
In this module you will apply your understanding of relationships among pressure, volume, temperature, and amount of gas to explain the function of common technologies.
The assessment for this module is combined with the unit assessment. For more information about the combined module and unit assessment, go to the Unit Assessment section.
1.2. In this Module
Module 3—Behaviour of Gases
In This Module
Lesson 1—Properties of Gases and Boyle’s Law
You will begin your study of gases by learning how to apply Boyle’s law—a mathematical relationship between the pressure and volume of a gas. You will also learn about pressure measurement and how to convert from one unit to another.
- What is pressure?
- How is pressure measured?
- How are pressure and volume of a gas related?
- How are pressure-volume relationships applied in technologies to support breathing?
Lesson 2—Kelvin Temperature, Charles’ Law, and the Combined Gas Law
While Boyle’s law establishes the relationship between the pressure and volume of a gas at a constant temperature, it may not be used if the temperature is changing. In this lesson you will learn that Charles’ law and the combined gas law may be used if the temperature of a gas changes. You will also learn about the Kelvin temperature scale and how to convert from degrees Celsius (ºC) to kelvin (K).
- What is the Kelvin temperature scale, and why is it used?
- What are the relationships among amount, pressure, volume, and temperature for a fixed amount of gas?
Lesson 3—The Law of Combining Volumes and Avogadro’s Theory
The kinetic molecular theory has important implications for gas laws. In this lesson you will examine Gay-Lussac’s law of combining volumes (not to be confused with the combined gas law) and Avogadro’s theory.
- Is it possible to predict the quantity of a gaseous substance involved in a chemical reaction?
Lesson 4—Molar Volume and the Ideal Gas Law
In the final lesson of this module you will learn about the ideal gas law. This law establishes the relationship between the molar amount of a gas and other gas properties, such as pressure, volume, and temperature.
- What is molar volume?
- What is the difference between a real gas and an ideal gas?
- What relationship exists between pressure, volume, temperature, and moles of gas?
 Module Assessment
Module Assessment
The Module Assessment and the Unit Assessment are combined for this module. Please see the Unit B Assessment document.
1.3. Lesson 1 Intro
Module 3—Behaviour of Gases
Lesson 1—Properties of Gases and Boyle’s Law
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
When you breathe, you do so unconsciously. Even when exercising, your body automatically increases the rate at which your lungs expand and the rate at which oxygen and carbon dioxide are exchanged by your body. The mechanics of breathing are often overlooked, but without the action of muscles that increase the size of your rib cage, air could not be drawn into your lungs.
The mechanics of breathing illustrates one of the crucial relationships in matter. Assisting someone to breathe, either by performing cardiopulmonary resuscitation (CPR) or by supplying them with a compressed gas, uses the same principle as breathing normally.
Essential Questions
- What is pressure?
- How is pressure measured?
- How are pressure and volume of a gas related?
- How are pressure-volume relationships applied in technologies to support breathing?
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
Save a copy of the Module 3: Lesson 1 Assignment to your course folder. Later in the lesson you will receive more information about how to complete this assignment and when to submit it to your teacher.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check and Try This questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answers to all the questions in this lesson and place those answers in your course folder. After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answers to all the questions in this lesson and place those answers in your course folder.
1.4. Page 2
Module 3—Behaviour of Gases
 Explore
Explore
 Read
Read
Gases exert a pressure, a force on surfaces they come in contact with. Atmospheric pressure is a measurement you often hear about since changes in pressure often bring a change to local weather.
To learn more about pressure and how the pressure exerted by a gas is measured, read “Pressure” on pages 148 and 149 in your textbook.
You may wish to prepare a list of the most important information in this reading. Compare your list with these summary notes on pressure. Save a copy of your notes or add the summary notes to your course folder for reference.
 Read
Read
You can see that a variety of units can be used to communicate a measurement of the pressure of a gas. In some situations it is necessary to convert between units. Equivalencies between the units are shown in the summary notes and in “Table 2: SI and Non-SI Units of Pressure” on page 149 of your textbook.
Carefully work through “COMMUNICATION example 1” on page 150 and the following examples.
Conversions between units may involve one set, or multiple equivalencies. You will need to use the equivalencies in your summary table.
A convenient method used in converting between units is to arrange the equivalencies into a fraction where the numerator and denominator are equal, but they are values in two different units. The numerator of the fraction should have the units that you want to convert to, and the denominator of your fraction should have the units you want to convert from. This arrangement will allow the unwanted units to cancel.
Example 1
- Convert 3.21 atm into units of kPa.
(Hint: 1 atm = 101.325 kPa)
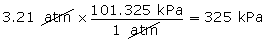
- Convert 0.78 bars into units of kPa.
(Hint: 1 bar = 100 kPa)
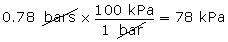
- Convert 670 mm Hg into units of torr.
(Hint: 1 torr = 1 mm Hg)
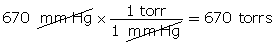
- Convert 167 kPa into units of atm.
(Hint: 1 atm = 101.325 kPa)
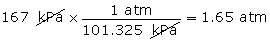
Equivalencies can be written between units shown on the table that are not already listed. For example, what is the equivalency between kPa and mm Hg? After you have command of these equivalencies, you can complete other unit conversions.
Example 2
- Convert 250 mmHg into units of kPa.
(Hint: 760 mm Hg = 1 atm = 101.325 kPa)
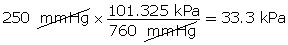
1.5. Page 3
Module 3—Behaviour of Gases
 Watch and Listen
Watch and Listen
Watch the following video to see an experimental illustration of the effect of pressure on the volume of a gas. You can toggle between the inquiry and explanatory audio tracks as well.
After you have watched the video, summarize what you observed. Where possible, use data from the video to support the points you make. Share your summary with your teacher.
 Read
Read
In the video you observed the effect of exerting a great pressure on the volume of a contained gas. But was there a pattern in changes you observed?
Read “Boyle’s Law” on page 151 in your textbook to learn more about this relationship. Carefully work through COMMUNICATION example 2 on page 151.
 Self-Check
Self-Check
SC 1.
Complete the following table by converting each measurement into the other four units.
kPa |
atm |
mm Hg |
torr |
bar |
|
4.05 |
|
|
|
50.0 |
|
|
|
|
|
|
|
|
1.33 |
|
|
|
770 |
|
|
|
616 |
|
|
SC 2. In Vancouver, where the air pressure is 100 kPa, a child is given a 2.5-L balloon. The family then drives to a higher elevation above sea level, where the air pressure is only 85 kPa. If the temperature is the same in both places, what is the volume of the balloon in the higher location?
SC 3. Complete “Practice” questions 6, 8, and 9 on page 152 in your textbook.
SC 4. A sample of gas is initially at a pressure of 53 kPa and has a volume of 1.8 L. Determine the new pressure if the volume of the gas decreases to 1.2 L.
SC 5. A sample of gas is initially at a pressure of 1.3 atm and has a volume of 2.3 mL. Determine the new volume if the pressure of the gas decreases to 1.1 atm.
SC 6. A sample of gas has a volume of 5.0 L. Determine the new volume of the gas if the pressure is tripled.
 Self-Check Answers
Self-Check Answers
SC 1.
kPa |
atm |
mm Hg |
torr |
bar |
410 |
4.05 |
3.08 x 103 |
3.08 x 103 |
4.10 |
50.0 |
0.493 |
375 |
375 |
0.500 |
133 |
1.31 |
998 |
998 |
1.33 |
103 |
1.01 |
770 |
770 |
1.03 |
82.1 |
0.811 |
616 |
616 |
0.821 |
SC 2. Use Boyle’s law.
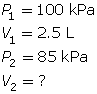
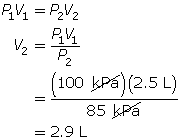
SC 3. Questions 6, 8 and 9 on page 152 of your textbook.
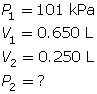
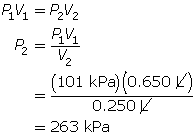
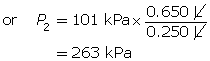
According to Boyle’s law, the final pressure of the air is 263 kPa.
- Pressure and volume are inversely related. If the pressure on the oxygen decreases, the volume should increase if the temperature and the chemical quantity of air remain constant.
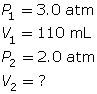
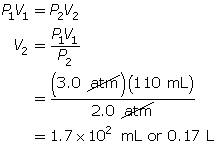
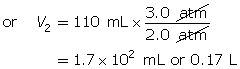
According to Boyle’s law, the final volume of the balloon will be 0.17 L.
SC 4. First, list all the variables.
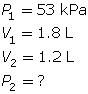
Now, apply Boyle’s law.
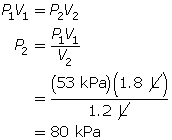
SC 5. First, list all the variables.
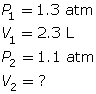
Now, apply Boyle’s law.
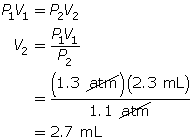
SC 6. Because the pressure is being tripled, call the initial pressure P and the final pressure 3P (3 times the initial pressure).
First, list all the variables.
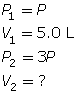
Now, apply Boyle’s law.
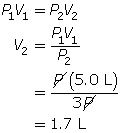
1.6. Page 4
Module 3—Behaviour of Gases
 Try This
Try This
The Diver—An Application of Boyle’s Law
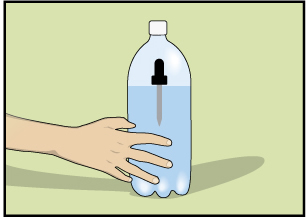
Step 1: Fill a glass with water, draw water into an eyedropper, and place the eyedropper into the glass of water. If it sinks, remove some of the water from the eyedropper. Continue to adjust the water in the eyedropper so that it just barely floats.
Step 2: Fill a clear plastic 1-L or 2-L pop container three-quarters full with water. Carefully place the eyedropper into the pop container without losing any of the water in the eyedropper.
Step 3: Tightly cap the pop container. (The eyedropper should be floating.)
Step 4: Squeeze the sides of the pop container. You should be able to make the eyedropper sink. Releasing the pressure on the sides of the pop container should make the eyedropper float. If this doesn't happen, you will have to adjust the amount of water in the eyedropper.
 Discuss
Discuss
Can you explain why the diver sinks when the pop bottle is compressed? Write an explanation, making explicit reference to Boyle's law and any observations of changes in pressure and volume you observed.
Post your explanation to the discussion area for your class. Read what other students have posted and consider which explanations make the most sense. You may wish to revise your explanation. Save your final version to your course folder.
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
You will complete the following lab as part of your Lesson 1 Assignment. Retrieve your saved copy of the Lesson 1 Assignment now.
 Lab: Boyle’s Law
Lab: Boyle’s Law
The following lab activity will enable you to complete a virtual experiment to investigate Boyle’s law. Ensure you read through the Background, Procedure, and Assignment before beginning the lab.
Complete the questions in Part 1 of the assignment. Save copies of the two graphs you draw for these data.
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
Complete Part 2 of the assignment. Save a copy of your work to your course folder.
1.7. Page 5
Module 3—Behaviour of Gases
 Reflect and Connect
Reflect and Connect
Respiratory therapists evaluate and care for patients who suffer from breathing problems due to chronic lung conditions, such as asthma and emphysema. They also work with people involved in motor vehicle collisions, people who have heart attacks, and premature infants.
Respiratory therapists continually work with gases, such as oxygen and carbon dioxide. They monitor lung capacity and work with patients to ensure they are breathing properly.
Read the transcript of an interview with a respiratory therapist to see how a knowledge of the pressure-volume relationship of gases is used in their work.
 Reflect on the Big Picture
Reflect on the Big Picture
Compressed Gases
Throughout this module you will be asked to consider technologies that apply the use of one or more of the gas laws. For your combined unit and module assessment, you will prepare a response on a technology that applies one or more of the gas laws. To prepare your response, consider the following description of compressed gases. Refer to the Unit Assessment section for more information.
Review the technologies described in the case study on “Compressed Gases” on pages 159 and 160 in your textbook.
Which of the technologies in this case study involves a pressure-volume relationship? Describe how this relationship is involved in the operation of the technology. Save your answer in your course folder.
 Assessment
Assessment
Submit the following assessment items to your teacher:
- Watch and Listen
- Discuss
- Module 3: Lesson 1 Assignment
1.8. Page 6
Module 3—Behaviour of Gases
 Lesson Summary
Lesson Summary
In this lesson you investigated the following questions:
- What is pressure?
- How is pressure measured?
- How are pressure and volume of a gas related?
- How are pressure-volume relationships applied in technologies to support breathing?
Gases are essential to life on Earth. In this lesson you learned about the mechanics of breathing and how this is an example of a pressure-volume relationship of a gas. You also learned how technologies to assist breathing must incorporate an understanding of pressure-volume relationships in order to function appropriately.
As you will see in the next lessons, an understanding of Boyle’s law is only one part of understanding the behaviour of gases.
1.9. Lesson 2 Intro
Module 3—Behaviour of Gases
Lesson 2—Kelvin Temperature, Charles’ Law, and the Combined Gas Law
 Get Focused
Get Focused
On a cold winter day in Alberta, temperatures may be around –30°C. On a hot summer day in Alberta, the temperature may be around 30°C. Is it easier to breathe in cold weather or hot weather? You probably find that it is more difficult to breathe when it is cold outside. This isn’t purely temperature dependent, however. Other factors, such as humidity, also affect breathing.
 Try This
Try This

Step 1: Inflate a balloon.
Step 2: Measure the size of the balloon.
Step 3: Place the balloon into a freezer (typically around –18°C), and leave it there for ten minutes.
Step 4: Remove the balloon from the freezer and quickly determine its size.
Does the balloon get bigger or smaller when it is cooled?
In Lesson 1 you learned that the pressure of a gas is the result of the collisions of the gas molecules against the surfaces of its container. Do you recall how temperature affects the motion of particles? How would this explain the change in the size of the balloon upon cooling and heating?
The investigation you completed identifies an important relationship between temperature and volume of a gas. This relationship was first described by Jacques Charles—a French chemist, physicist, and aeronaut. In 1783, Charles made the first hot-air balloon using hydrogen gas and ascended to a height of nearly 3 km. Charles’ work with hot-air balloons set the stage for his discovery of the relationship between temperature and volume.
In this lesson you will investigate this relationship, as well as consider the relationship of pressure, volume, and temperature for a fixed amount of gas.
Essential Questions
- What is the Kelvin temperature scale, and why is it used?
- What are the relationships among pressure, volume, amount, and temperature for a fixed amount of gas?
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
Save a copy of the Module 3: Lesson 2 Assignment to your course folder. Later in the lesson you will receive more information about how to complete this assignment and when to submit it to your teacher.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.10. Page 2
Module 3—Behaviour of Gases
 Explore
Explore
You may have observed a container rupture as a result of the expansion of the trapped gas inside. This event may occur if the gas is heated, for instance by the sun on a hot day or by exposure to another heat source.
 Read
Read
The kinetic molecular theory states that particles increase their rate of motion with increased temperature. How does this theory relate to the pressure and volume of a confined gas?
Read “The Relationship Between Temperature and Volume” on pages 152 to 153 and “Temperature Measurement Technologies” on pages 155 and 156 in your textbook. You will learn more about the absolute temperature scale and how it is related to the motion of particles. You will also find out why precise measurement is necessary for scientific investigation of gases.
 Self-Check
Self-Check
SC 1. Complete the following table.
Temperature (°C) |
Temperature (K) |
–38 |
|
|
358 |
104 |
|
|
413 |
173 |
|
 Self-Check Answers
Self-Check Answers
SC 1.
Temperature (°C) |
Temperature (K) |
–38 |
235 |
85 |
358 |
104 |
377 |
140 |
413 |
173 |
446 |
 Read
Read
In the Try This activity you completed earlier, you noticed that when the balloon cooled, it was reduced in size, or the volume the gas inside occupied, was less.
What would happen if you cooled or heated the gas inside the balloon and continued to measure its volume? Would you be able to observe a pattern?
Read “Charles’ Law” on pages 154 and 155 in your textbook. Carefully work through “COMMUNICATION example 3” and the “Learning Tip” in the margin on page 155.
Important: Remember that temperatures used in calculations must be in kelvin, not degrees Celsius.
 Self-Check
Self-Check
SC 2. A balloon containing 1.25 L of helium is heated and increases in temperature from 21°C to 87°C. If kept at constant pressure, what is the final volume of the helium in the balloon?
SC 3. A balloon has a volume of 55.0 L at 22.0°C. To what temperature does the balloon need to be raised to have a volume of 80.0 L at the same pressure?
SC 4. A gas has a volume of 3.30 L and a temperature of 25.0°C. The temperature of the gas is increased, and the new volume is 4.20 L. If the pressure is held constant, what is the new temperature of the gas?
SC 5. A gas is in a perfectly airtight metal cylinder with a movable top to allow for the expansion/compression of the gas. Charles’ law states that the ratio of volume to temperature for a gas is a constant, according to this formula:
![]()
Design an experiment to determine the value of k for the gas.
 Self-Check Answers
Self-Check Answers
SC 2. First, convert the temperatures to K.
21°C + 273 = 294 K
87°C + 273 = 360 K
Now, use Charles’ law.
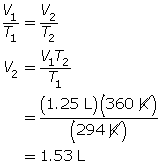
SC 3. First, convert the temperature to K.
22°C + 273 = 295 K
Now, use Charles’ law.
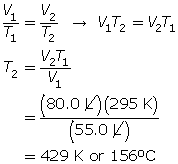
SC 4. First, list all the variables.
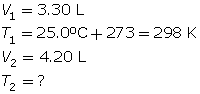
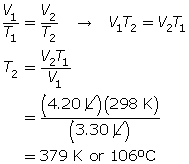
SC 5. A possible experiment is given.
Heat up the gas from 200 K to 300 K. Using increments of 10 K for each trial, measure the resulting volume of the gas. Plot the measurements on a graph with volume on the y-axis and temperature on the x-axis. Calculate the slope of the straight-line graph. This yields the value for k. (Recall that the equation V = kT is a straight line of the form y = mx.)
 Try This
Try This
For additional practice, try the following questions in your textbook:
- questions 11, 12, and 13 on page 154
- questions 14(a), 15, 16, and 17 on page 156
You can find the answers to these questions on page 783 in Appendix A of your textbook.
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
You will use the following lab to complete part of your Lesson 2 Assignment. Retrieve your saved copy of the Lesson 2 Assignment now.
1.11. Lab
Module 3—Behaviour of Gases
 Lab: Charles’ Law
Lab: Charles’ Law
The following lab activity will enable you to complete a virtual investigation of Charles’ law. Be sure you read through the Background, Procedure, and Assignment before beginning the lab.
Record the data collected from the experiment in Part 1 of the assignment, and complete the questions under Procedure 1 and Procedure 2. Save copies of the graphs you drew for the data. Save a copy of your work in your course folder.
 Read
Read
The combined gas law describes a relationship among pressure, volume, and temperature consistent with Boyle’s law and Charles’ law. Read “The Combined Gas Law” on page 156 to 158 in your textbook. Carefully work through “SAMPLE problem 4.1” and “COMMUNICATION example 4” on pages 157 and 158.
 Self-Check
Self-Check
SC 6. A gas has a volume of 32 L, a temperature of 22°C, and a pressure of 92 kPa. If the temperature is decreased to 19°C and the pressure is increased to 98 kPa, what is the new volume of the gas?
SC 7. The combined gas law is very useful, since it is possible to obtain both Boyle’s law and Charles’ law from this one formula.
- Explain how Boyle’s law may be obtained from the combined gas law.
- Explain how Charles’ law may be obtained from the combined gas law.
 Self-Check Answers
Self-Check Answers
SC 6. First, list all the variables.
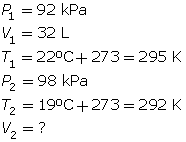
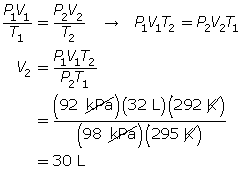
SC 7.
- If the temperature is held constant, T1 = T2 = T. Since T is identical on both sides of the equation, they will cancel, reducing the combined gas law to Boyle’s law.
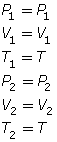
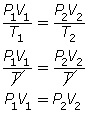
- If the pressure is held constant, P1 = P2 = P. Since P is identical on both sides of the equation, they will cancel, reducing the combined gas law to Charles’ law.
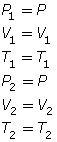
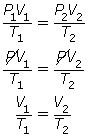
1.12. Page 4
Module 3—Behaviour of Gases
 Reflect and Connect
Reflect and Connect
High-Altitude Balloons
Throughout this module you will be asked to consider technologies that apply the use of one or more of the gas laws. For your combined unit and module assessment, you will prepare a response on a technology that applies one or more of the gas laws. To prepare your response, consider the following description of high-altitude balloons. Refer to the Unit Assessment document for more information.
The Breitling Orbiter 3 was the first hot-air balloon to complete a non-stop trip around the world. High-altitude ballooning involves a great deal of knowledge about gases and their behaviour. Use the Internet to research this topic by combining the search terms breitling, balloon, and design to learn more about the design of this balloon. Use your knowledge of the gas laws to explain how these design considerations are consistent with the relationships described in the gas laws you have studied.
Save a copy of your explanation in your course folder. You may wish to use this information when completing the unit assessment. For more information on the unit assessment, refer to the Unit Assessment document.
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
Retrieve the Module 3: Lesson 2 Assignment you saved to your course folder earlier in this lesson. Complete Part 2 of Lesson 2 Assignment, and save a copy of your work in your course folder.
Once you have completed all of the questions, submit your work to your teacher.
1.13. Page 5
Module 3—Behaviour of Gases
 Lesson Summary
Lesson Summary
In this lesson you investigated the following questions:
- What is the Kelvin temperature scale, and why is it used?
- What are the relationships among amount, pressure, volume, and temperature for a fixed amount of gas?
In this lesson you explored the Kelvin temperature scale and the basis of this scale—absolute zero. You discovered that when using Charles’ law and the combined gas law, Kelvin temperatures are needed. As such, you converted between degrees Celsius and kelvin.
You then discovered Charles’ law, which states as the temperature of a gas increases, the volume increases proportionally, provided that the pressure and chemical amount of gas remain constant.
You performed calculations and completed a virtual lab based on Charles’ law.
Finally, you combined Charles’ law with Boyle’s law to form the combined gas law and performed various calculations based on the resulting formula.
1.14. Lesson 3 Intro
Module 3—Behaviour of Gases
Lesson 3—The Law of Combining Volumes and Avogadro’s Theory
 Get Focused
Get Focused

© Lyle J. Anderson. Used with permission
High-altitude ballooning creates many unique situations that must be considered. One aspect is the quantity of available oxygen. Oxygen is necessary for the crew to survive, and at high altitudes in the atmosphere, oxygen is less plentiful. You may recall that hot air within the balloon is necessary for lift. The air inside the balloon is heated by a combustion reaction. How is this possible in the upper atmosphere with limited oxygen?
You may notice the large compressed gas cylinders along the side of the Breitling Orbiter 3, the first hot-air balloon to circle Earth without stopping. The gases in the Breitling orbiter balloon were heated using a combustion reaction involving propane and oxygen. When planning the flight, the balloonists needed to know how many canisters of each gas, propane and oxygen, to carry. How would knowledge of gas laws assist their efforts?
Essential Question
- Is it possible to predict the quantity of a gaseous substance involved in a chemical reaction?
 Assessment
Assessment
There is no Module 3: Lesson 3 Assignment.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.15. Page 2
Module 3—Behaviour of Gases
 Explore
Explore
 Read
Read
In Lessons 1 and 2 of this module you referred to the kinetic molecular theory of matter to help you visualize the behaviour of gas particles explained by the gas laws you have studied. This theory can be used to develop a model that explains the physical behaviour of gases. In the gas phase, all pure substances show remarkably similar physical behaviour.
In your experiments conducted thus far, the relationships among pressure, volume, and temperature by Boyle’s law and Charles’ law were verified with all the gases tested. What is true for nitrogen is true for oxygen, hydrogen, and any other gas. The behaviour of gases is independent of the type of gas present. The fact that different gases resemble each other so closely in their physical behaviour has some interesting results.
Read pages 163 and 164 in your textbook to review a summary of the application of concepts of the kinetic molecular theory to what you have learned thus far in this module.
 Self-Check
Self-Check
SC 1. Complete “Practice” question 1 (a) through (e) on page 164 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 1.
- The kinetic molecular theory states that an increase in temperature represents an increase in the average kinetic energy and greater average speed of the particles in the system. Higher speeds of the particles will result in more collisions with the container the gas is held in. The greater the number of collisions, the greater the pressure exerted by the gas.
- Reducing the volume of the container forces the same number of particles to be contained in a smaller space. Since the temperature remains constant, so would their average speed, but the particles would now be colliding against a smaller surface. The result is an increase in the number of collisions. The greater the number of collisions, the greater the pressure exerted by the gas.
- Particles of gases have greater space between them and greater freedom of motion. Therefore particles of other gases are able to move into the spaces between other gas particles to mix quickly.
- In a hydraulic system, force applied to one end needs to be transferred to the other end. Since gases are highly compressible, the force applied would not be efficiently transmitted as it would be with a liquid, which is not very compressible.
- Molecules are in constant motion, and therefore in a system containing many particles, they will collide often, changing the direction of colliding particles and making it very difficult for one particle to follow a straight path for a long distance. The bullet does not collide with an object that changes its path as readily as an oxygen or nitrogen molecule does.
1.16. Page 3
Module 3—Behaviour of Gases
 Read
Read
The kinetic molecular theory, Boyle’s law, and Charles’ law consider individual or mixtures of unreactive gases. What happens when gaseous substances mix and react? Is there evidence that shows how gaseous substances react with one another?
The Law of Combining Volumes

The law of combining volumes was observed by Joseph Gay-Lussac in 1809. This law states that when temperature and pressure are kept constant, the volumes of gaseous reactants and products in chemical reactions are always in simple ratios of whole numbers. An example is the formation of water, which always requires a ratio of 2 (hydrogen) : 1 (oxygen).
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Volume |
2.0 L |
|
1.0 L |
|
2.0 L |
Volumes of gases were measured because that was empirically possible. But how does a measure of the volumes of gases relate to the number of particles present? Is there a relationship between volume and number of particles in a system?
Avogadro’s Theory
In 1811, Amedeo Avogadro explained Gay-Lussac’s observations. Avogadro noticed the relationship between the ratio of gas volume and the coefficient ratio of a balanced equation.
The coefficient ratio of hydrogen gas to oxygen gas is also 2:1—the same as the gas volume ratio. Finally, there is Avogadro's Theory, which states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. An example is the formation of water.
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Balanced Equation |
2 H2(g) |
+ |
O2(g) |
→ |
2 H2O(g) |
Ratio |
2 |
|
1 |
|
2 |
Volume |
2.0 L |
|
1.0 L |
|
2.0 L |
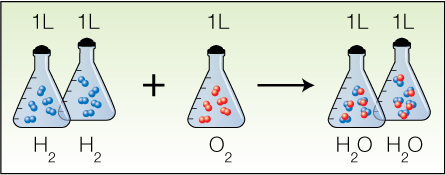
Since equal volumes contain equal numbers of gas molecules, a chemical equation in which all the substances are in the gas phase can be read two ways: as moles of each substance involved, or as litres of each substance involved.
It is important to note that the ratio describes the comparative quantity of each substance in the balanced equation, not the exact quantity. In other words, it does not always have to be 2.0 L of hydrogen, 1.0 L of oxygen, and 2.0 L of water. You must, however, always have twice as much hydrogen as oxygen during the formation of water.
Example
Complete the following table.
Word Equation |
hydrogen |
+ |
oxygen |
→ |
water |
Balanced Equation |
2 H2(g) |
+ |
O2(g) |
→ |
2 H2O(g) |
Ratio |
2 |
|
1 |
|
2 |
Volume |
|
|
225 mL |
|
|
According to the ratio, there is twice as much hydrogen as oxygen. Therefore, the volume of hydrogen must be 2 × 225 mL = 450 mL. Similarly, there is twice as much water as oxygen, which equates to 2 × 225 mL = 450 mL.
For additional clarification of this important law, read “Explaining the Law of Combining Volumes” on pages 164 to 166 in your textbook. Carefully work through “SAMPLE problem 4.2” and the “COMMUNICATION example” in this section.
You may wish to listen to the audio explanation of “SAMPLE problem 4.2.”
1.17. Page 4
Module 3—Behaviour of Gases
 Self-Check
Self-Check
SC 2. Complete the following table.
Word Equation |
hydrogen |
+ |
chlorine |
→ |
hydrogen chloride |
Balanced Equation |
H2(g) |
+ |
|
→ |
2 HCl(g) |
Ratio |
1 |
|
|
|
2 |
Volume |
|
|
1.1 L |
|
|
SC 3.
The Breitling Orbiter 3 uses the combustion of propane and oxygen to heat gases in the two chambers of the high-altitude balloon. Complete the following table to investigate the proportion by which propane and oxygen are used in a combustion reaction.
Word Equation |
propane |
+ |
oxygen |
→ |
carbon dioxide |
+ |
water vapour |
Balanced Equation |
|
+ |
5 O2(g) |
→ |
3 CO2(g) |
+ |
4 H2O(g) |
Ratio |
|
|
5 |
|
3 |
|
4 |
Volume |
|
|
|
|
|
|
80 mL |
SC 4. Use the law of combining volumes to find the volume of oxygen required for the complete combustion of 83.0 mL of propane. Assume the pressure and temperature are held constant.
SC 5. Butane gas is combusted to produce carbon dioxide and water vapour. If 2.4 mol of butane reacts, how many moles of water vapour will be produced?
SC 6. Complete question 5(a) on page 168 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 2.
Word Equation |
hydrogen |
+ |
chlorine |
→ |
hydrogen chloride |
Balanced Equation |
H2(g) |
+ |
Cl2(g) |
→ |
2 HCl(g) |
Ratio |
1 |
|
1 |
|
2 |
Volume |
1.1 L |
|
1.1 L |
|
2.2 L |
SC 3.
Word Equation |
propane |
+ |
oxygen |
→ |
carbon dioxide |
+ |
water vapour |
Balanced Equation |
C3H8(g) |
+ |
5 O2(g) |
→ |
3 CO2(g) |
+ |
4 H2O(g) |
Ratio |
1 |
|
5 |
|
3 |
|
4 |
Volume |
20 mL |
|
100 mL |
|
60 mL |
|
80 mL |
SC 4. First, write the balanced chemical formula.
C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g)
The ratio of oxygen to propane is: 5 molOxygen : 1 mol propane or
5 mLOxygen : 1 mL propane if the gases are at the same temperature and pressure.
Therefore, 83 mLpropane ( 5 mLoxygen / 1 mLpropane) = 415 mLoxygen
SC 5. First, write the balanced chemical formula.
2 C4H10(g) + 13 O2(g) → 8 CO2(g) + 10 H2O(g)
The ratio of water vapour to butane is: 10 molwater vapour : 2 mol butane
Therefore, 2.4 moLbutane ( 10 moLwater vapour / 2 moLbutane) = 12moLwater vapour
SC 6. Question 5 (a) on page 168 of your textbook.
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
V=100 L V = ? V= ? V= ?
Voxygen = 100Lammonia (5 Loxygen / 4 Lammonia) = 125 Loxygen
Vnitrogen monoxide = 100Lammonia (4 Lnitrogen monoxide / 4 Lammonia) = 100 Lnitrogen monoxide
Vwater vapour = 100Lammonia (6 Lwater vapour / 4 Lammonia) = 150 Lwater vapour
1.18. Page 5
Module 3—Behaviour of Gases
 Reflect on the Big Picture
Reflect on the Big Picture
High-Altitude Balloons
Throughout this module you will be asked to consider technologies that apply the use of one or more of the gas laws. For your combined unit and module assessment, you will prepare a response on a technology that applies gas laws. To prepare your response, consider the following information relating to the gases necessary for high-altitude flights. Refer to the Unit Assessment document for more information.
Large compressed gas cylinders containing oxygen, and others containing propane, are situated along the side of the Breitling Orbiter 3. A combustion reaction involving these two gases is used to heat the gases within the balloon. When planning the flight, the balloonists needed to know how many canisters of each gas, propane and oxygen, to carry.
Use your knowledge of the gas laws to explain how the law of combining volumes or Avogadro’s theory is incorporated in the design of the system to heat the gases in a high-altitude balloon.
Save a copy of your explanation in your course folder. You may wish to use this explanation when completing the unit assessment. For more information on the unit assessment, refer to the Unit Assessment section.
 Assessment
Assessment
There is no Module 3: Lesson 3 Assignment.
In this lesson you continued to consider technologies that apply the use of one or more of the gas laws. Later you will be given instructions about submitting the combined unit/module assessment to your teacher.
1.19. Page 6
Module 3—Behaviour of Gases
 Lesson Summary
Lesson Summary
In this lesson you explored the following question:
- Is it possible to predict the quantity of a gaseous substance involved in a chemical reaction?
Joseph Gay-Lussac developed the theory that gases react in distinct proportions. When gases are at similar temperatures and pressures, the proportions are identical to the volumes of each gas involved in the reaction.
This theory of combining volumes of gases was further supported by Avogadro’s law that extends an understanding of this relationship to the moles of each substance involved. This law states that when temperature and pressure are kept constant, equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
With this information, you can analyze and predict the quantities of gases involved in chemical reactions. In Unit D you will further your understanding of quantitative relationships in chemical reactions.
1.20. Lesson 4 Intro
Module 3—Behaviour of Gases
Lesson 4—Molar Volume and the Ideal Gas Law

© 2008 Jupiterimages Corporation
 Get Focused
Get Focused
A flat tire can be a real problem when cycling. Not only does it take time to change the tire, but getting the inflation correct can be tricky. Riding on an underinflated tire decreases efficiency. It may also decrease your ability to steer around hazards in your path. Pinching the tire is one way to check the inflation of a tire, but more often a pressure gauge is used to get an accurate reading. Inflating a tire to the appropriate pressure involves adding air, but what quantity of gas is necessary for proper inflation?
In Lesson 3 you began to investigate the relationship between the chemical quantity of a gas (moles) and one of its measurable properties (volume). In Lessons 1 and 2 you investigated relationships between volume and pressure and between volume and temperature.
There is also the empirical relationship you may have already observed—adding more gas (increasing moles) will increase the pressure exerted by gas.
How can all of these relationships be combined to better explain and predict the relationship between quantity of gas and its measurable properties?
Essential Questions
-
What is molar volume?
-
What is the difference between a real gas and an ideal gas?
-
What relationship exists between pressure, volume, temperature, and moles of gas?
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
Save a copy of the Module 3: Lesson 4 Assignment to your course folder. You will receive more information about how to complete this assignment later in this lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.21. Page 2
Module 3—Behaviour of Gases
 Explore
Explore
Molar Volume of Gases
Avogadro’s theory states that equal volumes of any gas, measured at constant temperature and pressure, contain equal numbers of molecules. But how many molecules are there?
From your previous learning in science you know that chemical quantities are expressed as moles. A mole is defined as 6.02 × 1023 atoms, molecules, or particles. Therefore when you indicate that a mole of propane combusts, you are also saying that 6.02 × 1023 molecules of propane combust. To make measuring a quantity of a gas easier, we often use volumes—so what volume does one mole of propane, or any other gas, occupy?
It should not surprise you that the molar volume for all gases is dependent upon the temperature and pressure of the gas. Therefore, there are two common temperature/pressure sets:
Set |
Conditions |
standard temperature and pressure (STP) |
0°C and 101.325 kPa |
standard ambient temperature and pressure (SATP) |
25°C and 100 kPa |
At these conditions a mole of any gas has the following volumes:
STP 22.4 L/mol.

This means that one mole of gas at STP constitutes about 11 two-litre pop bottles!
SATP 24.8L/mol
If the conditions are changed to SATP, a higher temperature, and a slightly lower pressure, the volume occupied by the same number of particles of gas is 24.8 L/mol.

This means that one mole of gas at SATP constitutes over 12 two-litre pop bottles!
Volumes are used when dealing with gases because measuring the mass of a gas is just too hard! Knowing the molar volume allows you to calculate the number of moles of a gas or the volume of gas according to this formula:
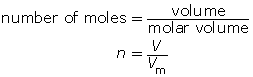
Example 1
How many moles of oxygen are available for a combustion reaction in a volume of 5.6 L at STP?
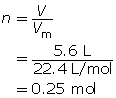
What volume is occupied by 0.024 mol of carbon dioxide gas at SATP?
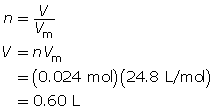
1.22. Page 3
Module 3—Behaviour of Gases
 Read
Read
For clarification of molar volume of gases, read the information on pages 169 and 170 in your textbook. You will also find out about the calculations involved in converting a volume of gas at one of the standard conditions into moles of gas.
 Self-Check
Self-Check
SC 1. Hydrogen gas can be used in weather balloons because of its low density. The volume occupied by 4.50 mol of hydrogen at SATP is
- 109 L
- 111 L
- 112 L
- 113 L
SC 2. Hydrogen gas can be used in weather balloons because of its low density. The volume occupied by 4.50 mol of hydrogen at STP is
- 99.1 L
- 101 L
- 102 L
- 112 L
SC 3. Nitrogen gas is an important gas in artificial breathing apparatus. The number of moles of nitrogen gas in 100 L of pure nitrogen at STP is
- 4.46 mol
- 4.03 mol
- 3.81 mol
- 2.76 mol
SC 4. Nitrogen gas is an important gas in artificial breathing apparatus. The number of moles of nitrogen gas in 100 L of pure nitrogen at SATP is
- 4.46 mol
- 4.03 mol
- 3.81 mol
- 2.76 mol
 Self-Check Answers
Self-Check Answers
SC 1. C
SC 2. B
SC 3. A
SC 4. B
In some situations the mass of the gas is able to be measured or is communicated. From your study of gases in this unit, you will understand why a value for mass is often converted into a volume for that gas. What calculation is necessary for this conversion?
Molar Mass
In previous science courses you learned how to calculate moles of a substance from mass. Molar mass is the value used to convert mass to moles. Follow the example below to review this conversion.
Example 2
Carbon dioxide is a greenhouse gas. Convert 1.5 kg of carbon dioxide into moles.
Step 1: Write the chemical formula for carbon dioxide.
CO2
Step 2: Determine the molar mass of carbon dioxide. The molar mass of each element is found on the periodic table.
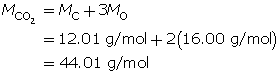
Step 3: Determine the number of moles.
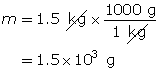
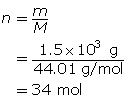
You can combine the two formulas for number of moles, n, to calculate the volume of gas that is available from a known mass of a substance. This is done as follows:
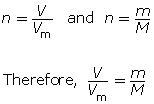
Example 3
What volume does 1.5 kg of carbon dioxide gas occupy at SATP?
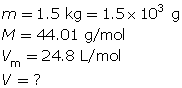
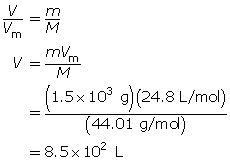
 Self-Check
Self-Check
SC 5. How many grams of oxygen gas are present in a 5.00-L container at STP?
SC 6. At SATP, what volume will 84.7 g of argon gas occupy?
 Self-Check Answers
Self-Check Answers
SC 5.
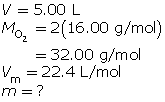
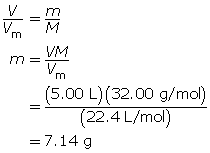
SC 6
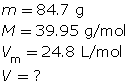
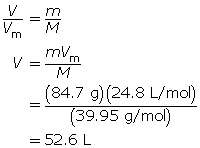
1.23. Page 4
Module 3—Behaviour of Gases
 Read
Read
Real vs. Ideal Gases
In Lesson 1 you completed a lab investigating the relationship between pressure and volume of two gases. Did you observe any difference in the way the gases behaved? If you look back on your data and graphs, you will notice that the data collected for the two gases are almost identical.
This lab demonstrated that as long as temperature was constant, and the gases were compressed to similar volumes, they produced the same pressure. As you learned in this module, the pressure exerted by a gas is a direct result of its motion. When gases produce equal pressures when all other conditions are similar, it means that the degree of motion of the two gases is the same.
In Unit A you learned about intermolecular forces and how attractions between atoms and molecules in a system can influence boiling point. You might ask whether the difference in intermolecular forces between these two gases tested would alter their behaviour and cause a difference in the observed pressure of each gas.
Clearly the data from your experiment shows this is not the case, and that the gases are behaving independent of these kinds of forces under the conditions of the experiment. When gases do not appear to be influenced by forces between them, along with other characteristics, they exhibit ideal behaviour.
Read “The Ideal Gas Law” on pages 172 through 174 in your textbook. You will find out what other conditions make the behaviour of a gas “ideal.” In this section you will also learn about the development of the ideal gas law, a relationship among all the variables you have studied in this unit: pressure, volume, temperature, and moles of gas.
Carefully work through “SAMPLE problem 4.4,” the “COMMUNICATION example” on page 174, and the following examples.
Example 4
How many grams of oxygen gas are there in a 60.0-L tank at 25.0°C when the pressure in the tank is 12.2 MPa (MPa = megapascals)?
First, determine the number of moles using the ideal gas law.
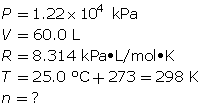
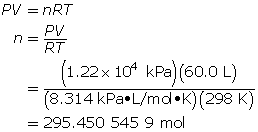
Now, determine the number of grams of oxygen.
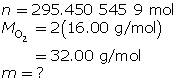
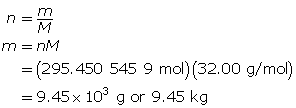
The mass of O2(g) is 9.45 × 103 g or 9.45 kg.
Example 5
Experiments show that a reaction will produce 12.7 g of CO2(g). What volume of CO2(g) should be expected at a new temperature of 40.0°C and 120.0 kPa?
First, find the number of moles of carbon dioxide gas.
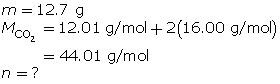
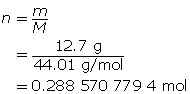
Now, determine the volume using the ideal gas law.
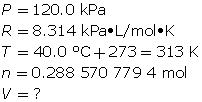
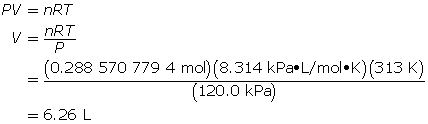
A volume of 6.26 L of CO2(g) should be expected.
As you can see by these examples, using the ideal gas law to predict the pressure, volume, or mass of gas can be very useful. One of the most practical reasons for using this relationship is to ensure the safety of people working with compressed gases and to ensure that the capacity of containers is not exceeded.
 Self-Check
Self-Check
SC 7. What mass of neon gas should be introduced into an evacuated 0.88-L tube to produce a pressure of 90 kPa at 30°C?
SC 8. When an air bag is activated in a collision, sodium azide rapidly decomposes to produce nitrogen gas. Chemical engineers carefully choose the quantity of sodium azide to produce the required chemical quantity of nitrogen gas. Use the ideal gas law to predict the chemical quantity of nitrogen gas required to fill a 60-L air bag at a pressure of 233 kPa and a temperature of 25°C.
SC 9. At what temperature does 10.5 g of ammonia gas exert a pressure of 85.0 kPa in a 30.0-L container?
SC 10. Use the ideal gas law to determine three ways to reduce the volume of gas in the shock absorber (cylinder and piston) of an automobile.
SC 11. A 1.49-g sample of a pure gas occupies a volume of 981 mL at 42.0°C and 117 kPa.
- Determine the molar mass of the compound.
- If the chemical formula is known to be XH3, identify element X.
 Self-Check Answers
Self-Check Answers
SC 7. First, find the number of moles using the ideal gas law.
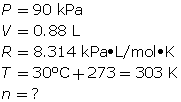

Finally, determine the mass of the neon.
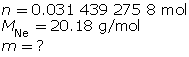
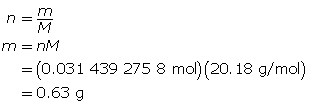
There should be 0.63 g of neon introduced to the tube.
SC 8.
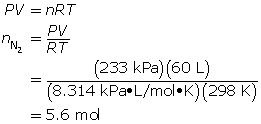
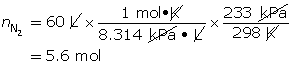
To fill the air bag, 5.6 mol of nitrogen gas is needed.
SC 9. First, determine the number of moles of ammonia.
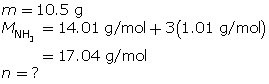
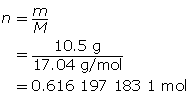
Now, calculate the temperature using the ideal gas law.
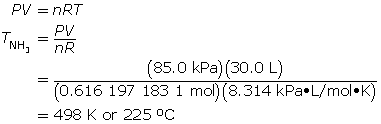
SC 10. The volume of a gas in an air (pneumatic) shock absorber can be reduced by decreasing the chemical amount of gas, by lowering the temperature, or by raising the pressure.
SC 11.
- First, determine the number of moles using the ideal gas law.
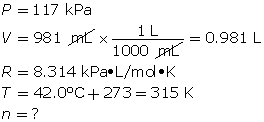

Now, determine the molar mass.
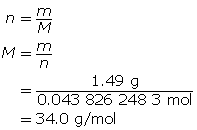
-
Since the formula is XH3, the molar mass of the three hydrogen atoms can be subtracted from the molar mass of the compound.
34.0 g/mol − 3.03 g/mol = 31.0 g/mol
This is the molar mass of the missing element. The element that has the molar mass closest to 31.0 g/mol is phosphorus. Therefore, the formula for the compound is PH3.
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
You will use the following lab to complete part of your Lesson 4 Assignment.
1.24. Lab
Module 3—Behaviour of Gases
 Lab: Molar Volume of an Ideal Gas
Lab: Molar Volume of an Ideal Gas
In chemistry, solving mathematical and scientific problems is often attempted in a laboratory. By using appropriate glassware, chemicals, equipment, and technology, you can complete experiments, record data, perform calculations based on the data, and draw conclusions.
The following lab activity will enable you to complete a virtual experiment to verify the molar volume of an ideal gas. At STP, the molar volume of a gas is 22.4 L/mol; and at SATP, the molar volume of a gas is 24.8 L/mol. This lab offers you an opportunity to test the molar volume of a gas with a different set of conditions, formulate a hypothesis, and test the results in the virtual lab.
Read through the Background, Procedure, and Assignment before beginning the lab.
Retrieve a copy of the Module 3: Lesson 4 Assignment. Complete Part 1; then save a copy of the assignment to your course folder.
1.25. Page 6
Module 3—Behaviour of Gases
 Reflect and Connect
Reflect and Connect
Compressed Carbon Dioxide Cartridges

Throughout this module you are asked to consider technologies that apply the use of one or more of the gas laws. For your combined unit and module assessment, you will prepare a response on a technology that applies gas laws. To prepare your response, consider the following information on compressed carbon dioxide cartridges. Refer to the Unit Assessment section for more information.
Compressed carbon dioxide cartridges are a popular alternative to a hand pump for many cyclists. Since the size of the tires and the suggested optimal inflation pressure varies between mountain bikes and road bikes, different sizes of cartridges are available. Each size of cartridge has a different mass of carbon dioxide inside.
Describe advantages and disadvantages of the use of compressed carbon dioxide gas cartridges in place of a hand pump to inflate bicycle tires. Make sure your list addresses at least three different perspectives.
Save a copy of your list in your course folder. You may wish to use this explanation when completing the unit assessment. For more information on the unit assessment, refer to the Unit Assessment section.
 Reflect on the Big Picture
Reflect on the Big Picture
Propane Cylinders

© 2008 Jupiterimages Corporation
Throughout this unit you have been asked to consider technologies that apply the use of one or more of the gas laws. Consider the use of propane cylinders.
How does a technician at a filling station know when a propane cylinder is full? Can you think of how the ideal gas law applies to the filling of propane cylinders, like the cylinders used with many gas barbeques? How is the safety of gas cylinders determined at a filling station? Does this system have merit?
You may wish to use your response to this question as your module and unit assessment. Refer to the Unit Assessment document for more information.
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
Retrieve the Module 3: Lesson 4 Assignment you saved to your course folder earlier in this lesson. Complete Part 2, and save a copy of your answers to your course folder.
Once you have completed all of the questions, submit your work to your teacher.
1.26. Page 7
Module 3—Behaviour of Gases
 Lesson Summary
Lesson Summary
In this lesson you investigated the following questions:
- What is molar volume?
- What is the difference between a real gas and an ideal gas?
- What relationship exists between pressure, volume, temperature, and moles of gas?
1.27. Module Summary/Assessment
Module 3—Behaviour of Gases
 Module Summary
Module Summary
In this module, you investigated the following question:
- What is the relationship among pressure, temperature, volume, and amount of a gas?
By performing experiments investigating the relationships among pressure, volume, and temperature, you confirmed the relationships described by Boyle’s law and Charles’ law.
You also investigated the influence of the amount of gas in a system on its pressure and volume, a relationship described by the ideal gas law.
In addition you learned how gases are used in a variety of technologies and how the principles that allow these technologies to operate are designed with a knowledge of the gas laws.
 Module Assessment
Module Assessment
The module and unit assessments are combined. For more information on the combined module and unit assessment, go to the Unit Assessment section.
1.28. Module Glossary
Module 3—Behaviour of Gases
Module Glossary
There is no glossary for this module.