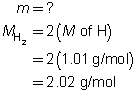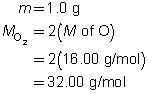Module 6 Intro
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 SS |
| Book: | Module 6 Intro |
| Printed by: | Guest user |
| Date: | Wednesday, 24 December 2025, 10:31 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 6 Intro
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1 Intro
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Page 6
- 1.9. Page 7
- 1.10. Lesson 2 Intro
- 1.11. Page 2
- 1.12. Page 3
- 1.13. Lab
- 1.14. Page 5
- 1.15. Page 6
- 1.16. Lesson 3 Intro
- 1.17. Page 2
- 1.18. Lab
- 1.19. Page 4
- 1.20. Page 5
- 1.21. Page 6
- 1.22. Lesson 4 Intro
- 1.23. Page 2
- 1.24. Lab
- 1.25. Page 4
- 1.26. Page 5
- 1.27. Module Summary/Assessment
- 1.28. Module Glossary
1. Module 6 Intro
Module 6—Stoichiometry
Module Introduction
During your study in this course you have seen that chemical reactions are important to the function of the human body, to interactions in the biosphere, and to many technologies used today.
In this module you will study chemical reactions from a different perspective by looking at the quantities of each substance involved in the reaction. Investigating quantitative relationships involves asking questions like
-
How much will react?
-
How much will be produced by the reaction?
-
How complete was the reaction?
Although understanding the quantity of matter involved in a chemical process is one of the more obvious aspects to consider when studying chemistry, you can only begin to ask it now. This unit will test your ability to manage and manipulate numerical data. You will also use numbers to help you keep track of the quantities of matter you are investigating.
Think about the following questions as you complete this module:
-
How do scientists and engineers use mathematics when analyzing chemical change?
-
How is a balanced chemical equation used to predict quantities of species involved in a chemical process?
1.1. Big Picture
Module 6—Stoichiometry
 Big Picture
Big Picture

Photo courtesy NASA/JSC
Have you ever had the opportunity to watch a launch of the Space Shuttle in person? The ability to launch rockets that leave Earth’s surface and travel into space is one of humankind’s greatest achievements.
The video below provides a close-up of a Space Shuttle launch from a unique perspective. This video comes from cameras located at various positions on the solid rocket booster engines, which are the long white cylinders that are located on each side of the large, orange main fuel tank. The table that appears below the video provides more information about what you are seeing in the video.
Time on Video (minutes) |
Event |
Camera Position on Solid Rocket Booster Engine |
0:00 to 1:00 |
Separation of solid rocket boosters and re-entry |
Bottom of left-side solid rocket booster looking upward |
1:01 to 2:48 |
Launch and separation |
Top of left-side solid rocket booster looking downward |
2:49 to 3:40 |
Separation of solid rocket boosters and re-entry |
Bottom of right-side solid rocket booster looking upward |
3:41 to 4:50 |
Launch and separation |
Top of right-side solid rocket booster looking downward |
4:50 to 6:00 |
Separation of solid rocket engines |
Midway along solid rocket booster engines |
Extremely vigorous chemical reactions take place in the rocket engines of the Space Shuttle. The solid rocket booster engines use one type of fuel, whereas the reaction that occurs in the main engines uses hydrogen and oxygen gases, which are contained in the large orange tank attached to the shuttle. How much hydrogen and oxygen is necessary to propel the Space Shuttle into space? Is a similar quantity of each gas required? How can the balanced chemical equation (shown below) of the reaction that occurs in the main engines provide you with information about the quantity of each gas required to perform this operation?
2 H2(l) + O2(l) → 2 H2O(g)
Examining chemical reactions using stoichiometry—an approach that considers the quantities of substances involved in a reaction—will be helpful. In order to learn about stoichiometry you will need to remind yourself of how to write, balance, and classify chemical reactions so that you can use them to interpret a chemical system. In this module you will learn to calculate the quantities of reactants and products involved in a chemical process, along with using calculations of percent yield to analyze the results of experiments you carry out.
By the end of this module you will have a better appreciation for stoichiometry. Whether you are planning to launch a rocket into space and support humans during spaceflight, or to experiment with a recipe when preparing food, a knowledge of stoichiometry is essential.
1.2. In this Module
Module 6—Stoichiometry
In This Module
Lesson 1—Chemical Equations
The focus of this lesson is to review the main types of chemical reactions and how to write balanced chemical equations. You will learn about what balancing means in terms of quantities of substances involved in a chemical reaction. The concepts covered in this lesson are critical to what you will learn in the remaining lessons.
-
What information about a chemical system is contained within a balanced chemical equation?
-
What information about a chemical system is not contained within a balanced chemical equation?
-
What is conservation of mass, and how is it demonstrated in a chemical equation?
Lesson 2—Gravimetric Stoichiometry
Stoichiometry is a method to predict the quantity of each substance that will be involved in a reaction. Are predictions made using this method verified by experimental results? In this lesson you will practise your skills and analyze data collected from an experiment. You will also discover how to evaluate a chemical reaction using both the theoretical and actual yield of a precipitate produced.
- How are predictions made about the masses of reactants and/or products involved in chemical reactions?
- How can you test the predictions made using the stoichiometric method?
- What is percent yield and how can it be determined?
Lesson 3—Gas Stoichiometry
In this lesson you will explore stoichiometry of chemical systems that involve the reactions of gases.
- How is the stoichiometric method applied to reactions that involve gases?
Lesson 4—Solution Stoichiometry
Do principles of stoichiometry apply to aqueous solutions? Are predictions made using this method verified by experimental results? In this lesson you will practise your skills and analyze data collected from experiments.
- How is the stoichiometric method applied to reactions that involve solutions?
 Module Assessment
Module Assessment
The assessment in this module consists of the following:
- Assignments for Lessons 1, 2, 3, and 4
- Module Assessment (Parts 1, 2, and 3)—Stoichiometry Calculator submitted to your teacher for review and comments.
- Module Assessment (Part 4)—Stoichiometry Calculator submitted to your teacher for a final grade.
The module assessment for this part of the course will be a spreadsheet that you design. You will complete different parts of the spreadsheet in each of the Reflect and Connect sections of the lessons in the module, and you will submit them to your teacher for feedback. More information about the spreadsheet can be found in the Module Assessment section.
Scoring Guide for the Stoichiometry Calculator
Score |
Criteria |
5 |
The design and function of the product is clearly explained. Operation of the Stoichiometry Calculator is straightforward and results in immediate, correct answers for all types of systems (mass, gasses, and solutions). Correct and logical algorithms are used.
|
4 |
The design and function of the product is well explained. Operation of the Stoichiometry Calculator is straightforward and results in immediate, correct answers for all types of systems (mass, gasses, and solutions). Correct and logical algorithms are used.
|
3 |
The design and function of the product is explained. Operation of the Stoichiometry Calculator results in immediate, correct answers for all systems (mass, gasses, and solutions). Correct and logical algorithms are used.
|
2 |
The design and function of the product is poorly explained. Operation of the Stoichiometry Calculator involves more than simple input of a few values. The use of the calculator does not consistently result in correct answers for all systems (mass, gasses, and solutions). Generally correct algorithms are used for most systems.
|
1 |
The design and function of the product has one or more serious flaws. The use of the calculator does not consistently result in correct answers for any systems, but evidence of an attempt is provided.
|
0 |
The response is not appropriate for a 20-level student. |
1.3. Lesson 1 Intro
Module 6—Stoichiometry
Lesson 1—Chemical Equations
 Get Focused
Get Focused

© webphotographeer/iStockphoto
Not all chemical reactions propel rockets into space. Energy needed to cook food comes from a combustion reaction. A combustion reaction is just one type of chemical reaction that you may recall from your previous science studies. What other types of reactions can you recall?
The focus of this lesson is to review how to write balanced chemical equations and to discover what balancing means in terms of the quantities of substances involved in a chemical process. The concepts covered in this lesson are critical to what you will learn in the remaining lessons of this module.
Essential Questions
- What information about a chemical system is contained within a balanced chemical equation?
- What information about a chemical system is not contained within a balanced chemical equation?
-
What is conservation of mass, and how is it demonstrated in a chemical equation?
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Save a copy of the Module 6: Lesson 1 Assignment to your course folder. You will receive more information about how to complete this assignment later in this lesson.
In this lesson you will also begin work on Module Assessment (Part 1)—Stoichiometry Calculator. What you develop in this lesson is the first part of a cumulative assessment for this module. You will submit the work you complete in this lesson to your teacher for feedback, and you will have the opportunity to revise and add to your Stoichiometry Calculator in the other lessons. Remember to keep a copy of your Stoichiometry Calculator in your course folder so you can revise it as you work through this module.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check and Try This questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answer to all the questions in this lesson and place those answers in your course folder.
1.4. Page 2
Module 6—Stoichiometry
 Explore
Explore
 Read
Read
Read “2.5 Classifying Chemical Reactions” on pages 58 and 59 of your textbook, and then turn to pages 62 and 63 and read about single replacement and double replacement reactions. To summarize what you have read, copy into your notebook the information in “Table 3: Reaction Generalizations” on page 63. Place a copy of your summary in your course folder so that it is convenient for you to access it.
 Self-Check
Self-Check
SC 1. For each of the reactants listed in question 9 on pages 66 and 67 of your textbook, identify the type of reaction that would occur. Justify your classification.
 Self-Check Answers
Self-Check Answers
SC 1.
|
Reaction Type |
Justification |
(a) |
simple decomposition |
Only one reactant, a compound, is undergoing change. |
(b) |
formation |
Both reactants are elements. |
(c) |
complete combustion |
One of the reactants is oxygen. |
(d) |
double replacement |
Both reactants are ionic compounds. |
(e) |
single replacement |
One reactant is an element and the other is an ionic compound. |
(f) |
complete combustion |
One of the reactants is oxygen. |
(g) |
simple decomposition |
Only one reactant is shown. |
formation |
Both reactants are elements. |
|
(i) |
double replacement |
Both reactants are ionic compounds. |
(j) |
double replacement |
Both reactants are ionic compounds. |
 Read
Read
Understanding the different types of chemical reactions is an important step in becoming able to predict the changes that may occur when reactants are combined. Review the products for each type of reaction listed in "Table 3 Reaction Generalizations" on page 63 of your textbook.
Predicting the products of a chemical reaction requires knowledge of the different types of chemical reactions as well as knowledge of how to write chemical formulas for the products. The information below summarizes some of major aspects for writing formulas for chemical compounds. You may wish to add this information to the summary of types of chemical reactions you completed earlier in this lesson.
Some things to be aware of when writing chemical formulas are as follows:
-
Some elements exist as molecular elements (e.g., H2, O2, Cl2, and P4).
-
The net charge of a compound must be zero.
-
Some metal ions can have more than one charge.
-
The state of any ionic compound in an aqueous system is (s) or (aq).
Review your understanding of these aspects by reading the following pages in your textbook:
-
“Names and Formulas of Ionic Compounds” on pages 29 to 31
-
“Molecular Elements” on page 33
-
how to use a solubility chart to determine the solubility of ionic compounds in aqueous systems and "Table 1: Solubility of Ionic Compounds at SATP–Generalizations," both on page 61
 Self-Check
Self-Check
SC 2. For each of the reactants listed in question 9 on pages 66 and 67 of your textbook, predict the products of the reaction by writing their chemical formulas and respective names.
 Self-Check Answers
Self-Check Answers
SC 2.
|
Chemical Formula(s) of Product(s) |
Name(s) of Products |
(a) |
K(s) + Cl2(g) |
solid potassium and chlorine gas |
(b) |
CuCl2(s) |
solid copper(II) chloride (Note: The 2+ charge is the most common charge for copper.) |
(c) |
CO2(g) + H2O(g) |
carbon dioxide gas and water vapour |
(d) |
AgCl(s) + NaNO3(aq) |
solid silver chloride and aqueous sodium nitrate |
(e) |
Al(NO3)3(aq) + Cu(s) |
aqueous aluminium nitrate and solid copper |
(f) |
CO2(g) + H2O(g) |
carbon dioxide gas and water vapour |
(g) |
Al(s) + O2(g) |
solid aluminium and oxygen gas |
FeBr3(aq) |
aqueous iron(III) bromide (Note: The 3+ charge is the most common charge for iron.) |
|
(i) |
Cu(OH)2(s) + NaNO3(aq) |
solid copper(II) hydroxide and aqueous sodium nitrate |
(j) |
H2O(l) + Ca3(PO4)2(s) |
liquid water and solid calcium phosphate |
1.5. Page 3
Module 6—Stoichiometry
 Read
Read
When you wrote chemical equations earlier in this course, you always ensured they were balanced. What does “balancing” an equation really mean? You may answer this question by stating that balancing an equation makes the quantity of each kind of atom equal with respect to the reactants and products sides of the equation.
law of conservation of mass: a law stating that in any physical or chemical system, the initial mass of the system will be identical to the final mass of the system
You may recall that balancing a chemical equation has a deeper significance—it describes how mass is conserved within a chemical system. The law of conservation of mass states that mass cannot be created nor destroyed. The atoms that exist today are essentially the same as those that existed millions of years ago; and over time, these atoms are recycled, breaking and forming bonds each time they react. The amount of matter at the beginning of a chemical reaction must be the same as the amount of matter at the end of the chemical reaction—all atoms and mass are accounted for.
To refresh your memory of how to balance chemical equations, read and work through the examples below. Additional information and examples appear on pages 52 and 53 in your textbook.
Example 1: Ammonia is produced by the combination of nitrogen and hydrogen gases. Balance the following chemical reaction for the formation of ammonia.
___ N2(g) + ___ H2(g) → ___ NH3(g)
Hydrogen is the atom with the largest subscript. Add a coefficient to the substances containing hydrogen on each side of the equation to balance the hydrogen atoms.
___ N2(g) + _3_ H2(g) → _2_ NH3(g)
Reactants |
Products |
3 × 2 = 6 H atoms |
2 × 3 = 6 H atoms |
Confirm that nitrogen atoms are balanced.
Reactants |
Products |
1 × 2 = 2 N atoms |
2 × 1 = 2 N atoms |
Recall that coefficients of 1 are not normally shown. Therefore, the balanced chemical equation is
N2(g) + 3 H2(g) → 2 NH3(g)
Example 2: Ethane is one component of natural gas. Balance the complete combustion reaction for ethane.
___ C2H6(g) + ___ O2(g) → ___ CO2(g) + ___ H2O(g)
Hydrogen is the atom with the largest subscript. Add a coefficient to balance the hydrogen atoms.
___ C2H6(g) + ___ O2(g) → ___ CO2(g) + _3_ H2O(g)
Add a coefficient to balance the carbon atoms.
___ C2H6(g) + ___ O2(g) → _2_ CO2(g) + _3_ H2O(g)
Add a coefficient to balance the oxygen atoms.
___ C2H6(g) + _3.5_ O2(g) → _2_ CO2(g) + _3_ H2O(g)
Recall that coefficients of 1 are not normally shown. Therefore, the balanced chemical equation is
C2H6(g) + 3.5 O2(g) → 2 CO2(g) + 3 H2O(g)
Note: If whole-number coefficients suit you better, multiply all coefficients by 2.
2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
Example 3: Solid silver forms as a precipitate when solid copper is placed in a solution of aqueous silver nitrate. Balance the reaction for this process.
___ Cu(s) + ___ AgNO3(aq) → ___ Ag(s) + ___ Cu(NO3)2(aq)
Treat polyatomic ions like a single unit. The nitrate ion is the group with the largest subscript. Add a coefficient to balance the atoms in the nitrate ions.
___ Cu(s) + _2_ AgNO3(aq) → ___ Ag(s) + ___ Cu(NO3)2(aq)
Add a coefficient to balance the silver atoms.
___ Cu(s) + _2_ AgNO3(aq) → _2_ Ag(s) + ___ Cu(NO3)2(aq)
Therefore, the balanced chemical equation is
Cu(s) + 2 AgNO3(aq) → 2 Ag(s) + Cu(NO3)2(aq)
 Self-Check
Self-Check
SC 3. Referring to your answers to question SC 2, write a balanced chemical equation for each reaction in question 9 on pages 66 and 67 of the textbook.
 Self-Check Answers
Self-Check Answers
SC 3.
- 2 KCl(s) → 2 K(s) + Cl2(g)
- Cu(s) + Cl2(g) → CuCl2(s)
- C5H12(g) + 8 O2(g) → 5 CO2(g) + 6 H2O(g)
- AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
- 2 Al(s) + 3 Cu(NO3)2(aq) → 2 Al(NO3)3(aq) + 3 Cu(s)
- C8H18(g) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(g)
- 2 Al2O3(s) → 4 Al(s) + 3 O2(g)
- 2 Fe(s) + 3 Br2(l) → 2 FeBr3(aq)
- Cu(NO3)2(aq) + 2 NaOH(aq) → Cu(OH)2(s) + 2 NaNO3(aq)
- 2 H3PO4(aq) + 3 Ca(OH)2(aq) → 6 H2O(l) + Ca3(PO4)2(s)
1.6. Page 4
Module 6—Stoichiometry
 Read
Read
When you first learned to balance equations, you may have used a table similar to the following to confirm your work.
Reaction |
2 H2(g) + O2(g) → 2 H2O(g) |
|
Atom |
Number of Atoms Before Reaction |
Number of Atoms After Reaction |
H |
4 (2 molecules of H2, 2 × 2H = 4) |
4 (2 molecules of H2O, 2 × 2H = 4) |
O |
2 (1 molecule of O2, 1 × 2O = 2) |
2 (2 molecules of H2O, 2 × 1O = 2) |
Read “2.3 Balancing Chemical Reaction Equations” on pages 51 to 53 of your textbook. What other ways can the information in a balanced chemical equation be interpreted? As you can see in the table, the coefficients used to balance a chemical equation can refer to the number (or quantity) of each type of particle. Coefficients also refer to chemical quantities, the number of moles—the quantity used to describe the chemical amount of a substance involved in a reaction.
mole ratio: a mathematical statement of the proportion of each substance involved in a chemical process relative to one another
stoichiometry: a method of predicting or analyzing the quantities of the reactants and products participating in a chemical process
One of the most important relationships conveyed in a balanced chemical equation is mole ratio. Mole ratio is a mathematical statement of the proportion of each substance involved in a chemical process relative to one another.
For example, in the reaction 2 H2(g) + O2(g) → 2 H2O(g), the mole ratio of hydrogen and oxygen is 2:1. This ratio shows that twice as many moles of hydrogen than oxygen are needed during the reaction. In terms of the quantity (moles) of hydrogen and oxygen needed to launch the Space Shuttle, failing to place enough hydrogen into the storage tank could prevent the Space Shuttle from reaching orbit.
This example identifies why knowledge of the proportions of substances involved in a chemical process, or stoichiometry, is important. Stoichiometry is a method of predicting or analyzing the quantities of the reactants and products participating in a chemical process. Play the following sound file to hear how this word is pronounced.
Mole ratios that exist in this reaction include the following:
2 H2(g) + O2(g) → 2 H2O(g) |
|
Relationship |
Mole Ratio |
hydrogen to oxygen |
2:1 |
hydrogen to water vapour |
2:2 (or 1:1) |
oxygen to water vapour |
1:2 |
Example 4: If 100 mol of oxygen were to be used in a test rocket engine, how could you use mole ratio to predict the quantities of hydrogen and water vapour involved in the reaction?
Mole ratios from the balanced chemical equation will be used to predict the quantity of hydrogen and oxygen involved when 100 mol of oxygen ![]() is reacted.
is reacted.
Mole ratio of
Predict the quantities of hydrogen gas and water vapour.
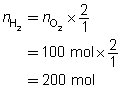
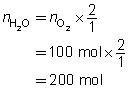
The predicted quantities of hydrogen and water vapour are each 200 mol.
 Self-Check
Self-Check
SC 4. The dehydration of sugar can be represented by the following balanced chemical equation.
C12H22O11(s) → 12 C(s) + 11 H2O(g)
Predict the quantity of carbon and water vapour produced by the dehydration of 15.0 mol of sucrose, C12H22O11(s).
SC 5. Predict the quantity of carbon dioxide and water vapour produced by the combustion of 398 mol of octane, C8H18(l). (This is approximately the quantity of gasoline in an automobile fuel tank.)
C8H18(g) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(g)
SC 6. Estimate the quantity of ammonium sulfide required to produce a solution containing 0.253 mol of dissociated sulfide ions.
(NH4)2S(aq) → 2 NH4+(aq) + S2−(aq)
 Self-Check Answers
Self-Check Answers
SC 4. C12H22O11(s) → 12 C(s) + 11 H2O(g)
List the known quantities and the mole ratios required.
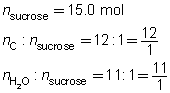
Predict the quantities of solid carbon and water vapour.
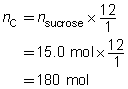
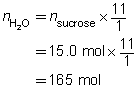
The predicted quantities of solid carbon and water vapour are 180 mol and 165 mol, respectively.
SC 5. C8H18(g) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(g)
Use the mole ratios to predict the desired quantities.
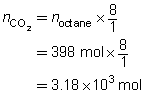
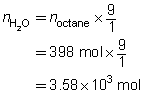
The predicted quantities of carbon dioxide and water vapour are 3.18 × 103 mol and 3.58 × 103 mol, respectively.
SC 6. (NH4)2S(aq) → 2 NH4+(aq) + S2–(aq)
Since the mole ratio is 1:1, the predicted quantity of ammonium sulfide is 0.253 mol.
 Read
Read
Examining the quantitative aspects of a chemical reaction can provide an interesting insight. For example, can you identify the significance of the large quantity of carbon dioxide produced by the combustion of octane in question SC 5? Why does there appear to be an increase to the number of moles of the carbon compounds in this reaction? As you will see in the remaining lessons of this module, quantitative relationships are very significant when they relate to technologies that rely on chemical reactions.
Thus far, you have discovered that a great deal of information is represented within a balanced chemical equation. What kind of information does a balanced chemical equation not provide? What assumptions are made when writing or discussing a process represented by a balanced chemical equation?
Read “Chemical Reaction Equations” on pages 278 to 281 of your textbook.
1.7. Page 5
Module 6—Stoichiometry
 Discuss
Discuss
Construct a two-column table. In the first column, in separate rows, list each of the four assumptions about written chemical reactions. In the second column, list examples of each assumption that you can identify from your experiences in this course and in other science courses.
Place a copy of your table into your course folder, and then post a copy to the discussion board. Read the postings of two other students and comment on the suitability of the examples they have used. You may wish to modify your table based on the examples and feedback you get from other students. Save any revised copies of your table in your course folder. Share your table with your teacher.
 Read
Read
net ionic equation: a type of balanced chemical equation that lists only the reacting particles
In Module 4, Lesson 1, you learned the effect water can have on dissolving ionic compounds. The process of dissociation creates separated ions, each with the possibility to collide with other ions and participate in a chemical reaction. To communicate which ions are participating in a chemical reaction, a unique type of balanced chemical equation is used—the net ionic equation. A net ionic equation is a type of balanced chemical equation that lists only the reacting particles.
Read “Net Ionic Equations” on pages 281 to 283 of your textbook. Work through the sample problem and examples.
 Self-Check
Self-Check
SC 7. Complete “Practice” questions 10, 11, and 13 on page 284 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 7. “Practice” questions 10, 11, and 13, page 284
-
Write the balanced chemical equation.
Pb(s) + 2 AgNO3(aq) → Pb(NO3)2(aq) + 2 Ag(s)
Now, write the total ionic equation.
Pb(s) + 2 Ag+(aq) + 2 NO3–(aq) → Pb2+(aq) + 2 NO3–(aq) + 2 Ag(s)
Cancel the ions that appear on both sides of the equation.
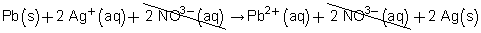
Therefore, the net ionic equation is
Pb(s) + 2 Ag+(aq) → Pb2+(aq) + 2 Ag(s)
- (a)
Balanced chemical equation: 3 CaCl2(aq) + 2 Na3PO4(aq) → Ca3(PO4)2(s) + 6 NaCl(aq)
Total ionic equation: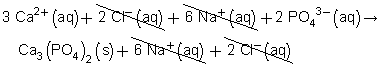
Net ionic equation: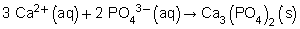
(b) The spectator ions are chloride and sodium.
-
(a)
Balanced chemical equation: HNO3(aq) + NaHCO3(aq) → NaNO3(aq) + H2CO3(aq)
Total ionic equation: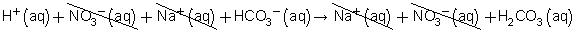
Net ionic equation: H+(aq) + HCO3−(aq) → H2CO3(aq)
(b) The spectator ions are nitrate and sodium.
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Complete the Module 6: Lesson 1 Assignment that you saved to your course folder at the beginning of this lesson. Submit your assignment to your teacher.
1.8. Page 6
Module 6—Stoichiometry
 Reflect and Connect
Reflect and Connect
 Module Assessment (Part 1)—Stoichiometry Calculator
Module Assessment (Part 1)—Stoichiometry Calculator
In this lesson you have learned to calculate the number of moles of an unknown substance by using a mole ratio. You may have identified that this calculation is one that you may be asked to perform many times. Our society routinely uses technology to perform many routine, or repetitive, tasks. When calculations are required, spreadsheets or computer programs are often used. In this exercise you will use your knowledge of stoichiometry to construct an automatic mole calculator that will calculate the moles for each of the substances listed in the balanced chemical equation. You will use the moles for one species and the coefficients provided in the balanced chemical equation. You will also write instructions on how to use your calculator.
Submit an electronic version of your calculator and the instructions for its use to your teacher along with a brief explanation of how it was constructed. Provide an example of how you tested the calculator to ensure that it works properly. Your teacher will review your work and provide feedback. You are welcome to use any feedback to modify and improve your work. Save copies of your work and the feedback from your teacher in your course folder. In the next lessons you will be asked to add other aspects to your Stoichiometry Calculator. At the completion of this module, you will submit your completed Stoichiometry Calculator and instructions as the Module Assessment.
 Reflect on the Big Picture
Reflect on the Big Picture
How does your understanding of stoichiometry help you understand a chemical system with an intended purpose? Earlier in this lesson you learned that the quantities of reactants involved in a chemical reaction can influence the quantities of products. You also learned that knowledge of the ratio by which substances react is important in the design of a reaction.
How is an understanding of stoichiometry used in cooking? In Module 5, Lesson 2, you experimented with baking soda, and you found that it would produce carbon dioxide gas with the addition of water.
Another commonly used baking product is baking powder, which contains sodium hydrogen carbonate and tartaric acid mixed together as solids. In what proportions must the components be present in order for baking powder to achieve its desired purpose? How does the design of baking powder represent an understanding of stoichiometry and provide convenience for people using the product? Place a copy of your answers in your course folder and send a copy to your teacher.
 Assessment
Assessment
Submit the following assessment items to your teacher:
-
Module 6: Lesson 1 Assignment
-
Discuss
-
Reflect on the Big Picture
-
Module Assessment (Part 1)—Stoichiometry Calculator
1.9. Page 7
Module 6—Stoichiometry
 Lesson Summary
Lesson Summary
In this lesson you explored the following questions:
-
What information about a chemical system is contained within a balanced chemical equation?
-
What information about a chemical system is not contained within a balanced chemical equation?
-
What is conservation of mass, and how is it demonstrated in a chemical equation?
You were introduced to stoichiometry, a mathematical process to determine quantities of substances involved in a chemical process. You learned that the information contained within balanced chemical equations provides information about chemical quantities, such as the number of moles of substances involved.
You discovered that the coefficients of a balanced chemical equation describe the proportions of each substance involved in the reaction, and how these proportions are an essential part of the predictions made using stoichiometry.
You learned that analyzing a system in terms of number of moles of substances does not allow you to comment on measurable quantities like mass or volume. In the lessons to come, you will extend your understanding of stoichiometry to use measurements of matter to predict the outcomes of a variety of chemical reactions.
Lesson Glossary
law of conservation of mass: a law stating that in any physical or chemical system, the initial mass of the system will be identical to the final mass of the system
mole ratio: a mathematical statement of the proportion of each substance involved in a chemical process relative to one another
net ionic equation: a type of balanced chemical equation that lists only the reacting particles
stoichiometry: a method of predicting or analyzing the quantities of the reactants and products participating in a chemical process
1.10. Lesson 2 Intro
Module 6—Stoichiometry
Lesson 2—Gravimetric Stoichiometry
 Get Focused
Get Focused
In Lesson 1 you learned that stoichiometry is a method to predict the quantities of reactants or products involved in a chemical reaction. Whether you plan to launch a rocket, and need to calculate oxygen to fuel ratios, or need to calculate the quantity of product that will result from a chemical reaction, a knowledge of stoichiometry is essential. In Lesson 1 you concentrated on the significance of ratios among the coefficients of different substances in the reaction to this method of calculation.
There are two major drawbacks to the predictions you have made thus far. First, you have only been able to consider chemical quantities of each substance (number of moles) involved. You already know that the number of moles is not a directly measurable quantity. Second, as is the case with all predictions, they need to be confirmed.
gravimetric analysis: the determination of masses of substances
In this lesson you will continue your study of stoichiometry by learning how to use the masses of the substances involved in your predictions. You will also perform an experiment to test predictions made using stoichiometry. By completing these activities, you will perform a gravimetric analysis. Gravimetric analyses involve the determination of masses of substances. Open the following sound file to hear how this word is pronounced.
Essential Questions
-
How are predictions made about the masses of reactants and/or products involved in chemical reactions?
-
How can you test the predictions made using the stoichiometric method?
-
What is percent yield and how can it be determined?
 Module 6: Lesson 2 Assignment
Module 6: Lesson 2 Assignment
Save Module 6: Lesson 2 Assignment to your course folder. You will receive more information about how to complete this assignment later in this lesson.
You will also continue to work on the Module Assessment (Part 2)—Stoichiometry Calculator. You will submit the work you complete in this lesson to your teacher for feedback, and you will have the opportunity to revise and add to it in other lessons. Remember to keep a copy of your Stoichiometry Calculator in your course folder so you can revise it as you work through this module.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.11. Page 2
Module 6—Stoichiometry
 Explore
Explore
 Read
Read
In Lesson 1 of this module you examined the chemical reaction that occurs in the main engines of the Space Shuttle.
2 H2(l) + O2(l) → 2 H2O(g)
From your work in Lesson 1, you identified important aspects of this balanced chemical equation, such as the mole ratio for hydrogen and oxygen. You would correctly interpret this ratio as meaning that twice as many moles of hydrogen than oxygen are needed for this reaction.
What would happen if a model for this reaction were to be planned using 2.0 g of hydrogen and 1.0 g of oxygen? Would the proper stoichiometric ratio for the reaction be met? Read and follow the calculations given in the table to answer this question.
Reactant |
H2(l) |
O2(l) |
Mass |
2.0 g |
1.0 g |
Molar Mass |
2.02 g/mol |
32.00 g/mol |
Number of Moles |
0.99 mol |
0.031 mol |
Mole Ratio of (H2:O2) |
32:1 |
|
Desired Mole Ratio |
2:1 |
|
* The desired mole ratio is derived from the balanced chemical equation. |
||
Compare the mole ratio calculated from the masses of each reactant with the desired mole ratio from the balanced chemical equation. Would the correct proportion of hydrogen be used in this model reaction? Can you provide an explanation as to why the correct proportion of these two reactants could not be met?
As you may have identified in the previous example, hydrogen and oxygen have significantly different molar masses. This was not accounted for when suggesting masses of each gas to use in the model reaction. As a result, there are very different numbers of moles for the two reactants. What effect would completing this reaction with these masses have?
yield of a reaction: a measured quantity of product obtained by a chemical reaction often expressed as a percentage of maximum yield
If the reaction were allowed to occur with the masses suggested, a large amount of hydrogen would not have reacted, simply because not enough oxygen was present. In Module 7 you will learn more about how using quantities of reactants that are not stoichiometric can affect the yield of a reaction. The yield of a reaction is a measured quantity of product obtained by a chemical reaction. It is often expressed as a percentage of maximum yield. You may have also thought that since all the hydrogen was not able to react, a much reduced quantity of energy would have been produced by the reaction as well.
What mass of hydrogen would be required to react with all the oxygen in the model reaction? How could you use your knowledge of stoichiometry to calculate this?
By examining this example, you have seen that making predictions using stoichiometry must always focus on the number of moles of each substance involved. Unfortunately, there is no device to measure the number of moles of each substance. Therefore, to complete predictions using stoichiometry when provided the mass of a substance involved, convert the mass into number of moles using this relationship:
![]()
Work through the following example to see how stoichiometric predictions can be made when you are provided with the mass of one reactant in a chemical reaction.
Example 1: For testing a rocket engine, what mass of hydrogen gas is required to completely react with 1.0 g of oxygen gas?
Step 1: Write the balanced chemical equation for the reaction.
2 H2(g) + O2(g) → 2 H2O(g)
Step 2: Organize the information provided.
2 H2(g) |
+ |
O2(g) |
→ |
2 H2O(g) |
|
|
|
|
|
Step 3: Calculate the number of moles of oxygen.
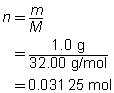
Step 4: Use the mole ratio between hydrogen and oxygen to determine the number of moles of hydrogen.
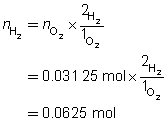
Step 5: Calculate the mass of hydrogen required.
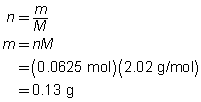
To completely react 1.0 g of oxygen gas, 0.13 g of hydrogen gas are required.
Read “Calculating Masses Involved in Chemical Reactions” on page 288 of your textbook. Then work through the “SAMPLE problem 7.2” and “COMMUNICATION example” on pages 288 and 289.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 9 to 14 on page 290 of your textbook.
 Read
Read
Read “Testing the Stoichiometric Method” on pages 290 and 291. Then read “Evaluation” on pages 791 and 792.
 Try This
Try This
Read “LAB EXERCISE 7.A: Testing the Stoichiometric Method” on page 291 of your textbook.
TR 1. Predict the mass of lead produced using the stoichiometric method.
TR 2. According to the evidence gathered, determine the actual mass of lead produced in this experiment.
TR 3. What is the percent difference between the experimental and predicted results?
Submit the answers to these questions to your teacher.
1.12. Page 3
Module 6—Stoichiometry
 Try This
Try This
Testing any predictions made using the stoichiometric method involves performing an experiment and comparing the prediction with the measurable quantity (result) obtained. In the following activities you will test the stoichiometric method using gravimetric analysis.
In the pre-lab component you will calculate the expected result for the experiment. Following the pre-lab, you will use a virtual lab investigation to perform an experiment. Part of your analysis of the investigation will require you to determine a percentage difference between the predicted and observed mass of product. For the purpose of this investigation and the equipment used, a percentage difference of less than 5% would support the hypothesis that predictions made using the stoichiometric method are supported by experimental evidence.

© Schaefer Elvira/shutterstock
Pre-lab: Decomposition of Malachite
Malachite is commonly used in jewellery. Malachite can react when heated. In this investigation you will heat malachite to test whether the predictions made using the stoichiometric method are confirmed by experimental data.
Note: The correct chemical formula for malachite is Cu(OH)2CuCO3(s), not what is stated in your textbook on page 288. The reaction of malachite when exposed to heat is as follows:
Cu(OH)2CuCO3(s) → 2CuO(s) + CO2(g) + H2O(g)

© Paul Hebditch/iStockphoto
TR 4. Predict the mass of copper(II) oxide that will remain if 2.000 g of malachite undergoes decomposition when heated.
Send a copy of your prediction to your teacher, and keep a copy for yourself so you can refer to it during the lab activity that follows.
1.13. Lab
Module 6—Stoichiometry
 Module 6: Lesson 2 Assignment
Module 6: Lesson 2 Assignment
You will complete the questions for this lab as your assignment for this lesson. When you are ready to answer the lab questions, open the Module 6: Lesson 2 Assignment that you saved to your course folder at the beginning of this lesson. Type your answers into this document, and save the completed copy to your course folder and submit a copy to your teacher.
 Lab: Decomposition of Malachite
Lab: Decomposition of Malachite
Problem
Are predictions made using the stoichiometric method supported by experimental evidence?
Purpose
The following virtual lab will test the prediction you made in the pre-lab exercise. Before beginning the lab, ensure you read through all parts of the laboratory, including the background, procedure, and assignment. As you read through the procedure, you may wish to make a data table to record your observations.
Decomposition of Malachite Virtual Lab
 Read
Read
Read “Applications of Stoichiometry” on page 292 of your textbook.
 Self-Check
Self-Check
SC 2. Complete questions 8 and 10 of “Section 7.2 Questions” on page 293 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 2. Section 7.2 Questions 8 and 10, page 293
- a.
2 AgNO3(aq)
+
Na2CrO4(aq)
→
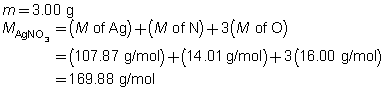
Ag2CrO4(s)
+
2 NaNO3(aq)
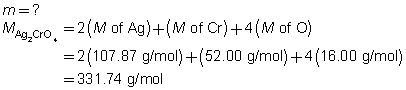
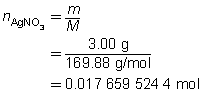
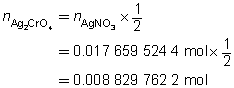
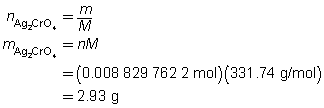
The mass of the precipitate is predicted to be 2.93 g.
b.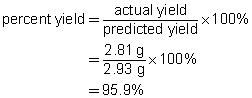
- a.
BaCl2(aq)
+
Na2SO4(aq)
→
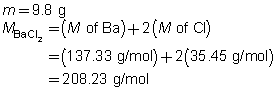
BaSO4(s)
+
2 NaCl(aq)
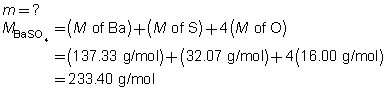
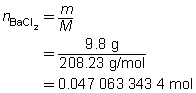
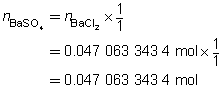
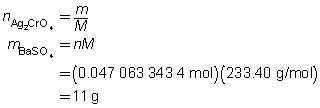
The mass of the precipitate is predicted to be 11 g.
b.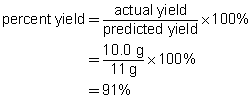
c. The percent yield suggests that minor mistakes were made during the experiment. These mistakes may have involved a loss of product while handling the precipitate or a loss of reactant during the preparation. The result is within the 5%–10% margin normally expected for school laboratory work.
1.14. Page 5
Module 6—Stoichiometry
 Reflect and Connect
Reflect and Connect
Module Assessment (Part 2)—Stoichiometry Calculator
Did you recently modify the Stoichiometry Calculator based on the comments from your teacher? Retrieve the latest version from your course folder. Now that you have learned to perform stoichiometric calculations with masses, modify your Stoichiometry Calculator to complete calculations involving masses as well as moles. Also, do not forget to update the instructions that accompany your Stoichiometry Calculator.
Submit the updated electronic version of your Stoichiometry Calculator and instructions to your teacher for feedback. As in the previous lesson, you will be allowed to modify your Stoichiometry Calculator using the feedback you get from your teacher. Remember to keep a copy of your Stoichiometry Calculator in your course folder so you can revise it as you work through this module.
 Reflect on the Big Picture
Reflect on the Big Picture

© Zsolt Nyulaszi/shutterstock
At the beginning of this lesson you examined how using appropriate quantities of hydrogen and oxygen can affect the products and usefulness of a chemical reaction. Knowledge of stoichiometry allows you to use appropriate quantities of reactants in order to maintain safety in many situations.
 Discuss
Discuss
Describe a situation where knowledge of stoichiometry is required. The situation you describe may be an industrial, commercial, or household process.
For your description, ensure that you properly cite any sources of information you use. Post your description to the discussion board. Read the descriptions of two other students. Provide comments on how to improve the clarity of the descriptions you read. You may revise your description using the comments provided by other students. Place a final copy of your description in your course folder and send a copy to your teacher.
 Assessment
Assessment
Submit the following assessment items to your teacher:
-
Module 6: Lesson 2 Assignment
-
TR 1, 2, 3, and 4
-
Discuss: Situation where knowledge of stoichiometry is required
-
Module Assessment (Part 2)—Stoichiometry Calculator
1.15. Page 6
Module 6—Stoichiometry
 Lesson Summary
Lesson Summary
In this lesson you investigated the following questions:
-
How are predictions made about the masses of reactants and/or products involved in chemical reactions?
-
How can you test the predictions made using the stoichiometric method?
-
What is percent yield and how can it be determined?
The procedure for calculating the masses of reactants or products in a chemical reaction is called gravimetric stoichiometry. There are four steps in solving any gravimetric stoichiometry problem:
Step 1: Write a balanced chemical equation, and list the given information—the masses and molar masses of the known and desired substances.
Step 2: Convert the mass of the known substance into number of moles.
Step 3: Use the mole ratio to convert the number of moles calculated in Step 2 into the number of moles for the desired substance.
Step 4: Calculate the mass of the desired substance using the number of moles calculated in Step 3.
You discovered that the stoichiometric method can be tested by comparing predicted values with data collected through experimentation. You also calculated the percent difference and found that percent difference is a useful way to determine the validity of predictions made using the stoichiometric method.
You also calculated percent yield. Percent yield is the ratio of the difference between the actual experimental quantity of product obtained and the maximum possible theoretical quantity of product (as determined by the stoichiometry method). In Lesson 3 you will use percent yield to assess the results of an experiment.
Lesson Glossary
gravimetric analysis: the determination of masses of substances
yield of a reaction: a measured quantity of product obtained by a chemical reaction, often expressed as a percentage of maximum yield
1.16. Lesson 3 Intro
Module 6—Stoichiometry
Lesson 3—Gas Stoichiometry
 Get Focused
Get Focused

Photo courtesy Kennedy Space Center/NASA
The hydrogen and oxygen that power the main engines of the Space Shuttle during launch are placed into the large orange external fuel tank mounted to the shuttle (as seen in this photo). The white ring that appears at the top of the external fuel tank is used to transfer hydrogen and oxygen (as liquids) into the tank.
In Lesson 2 you discovered that stoichiometry can be used to predict the mass of each of these substances that needs to be placed into the external fuel tank. During the combustion of hydrogen and oxygen, the temperature of the main engines of the shuttle can exceed 3300°C. At these conditions, the water produced by the reaction would exist as a gas. As you may recall from your study in Module 4, gases may fluctuate in volume in response to the temperature and pressure at which they are contained.
Predicting the quantity of a gas that is involved in a chemical reaction must still involve the number of moles of substance; but, in this case, the number of moles will be measured using the pressure, volume, and temperature of the gas. Can you recall a relationship that includes these four variables? How would you use this relationship when predicting the quantity of a gaseous substance involved in a chemical reaction?
In this lesson you will use stoichiometry to predict the quantities of substances in chemical reactions that involve gases.
Essential Questions
-
How is the stoichiometric method applied to reactions that involve gases?
![]() Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
Save a copy of the Module 6: Lesson 3 Assignment to your course folder. You will receive more information about how to complete this assignment later in the lesson.
You will also continue your work on Module Assessment (Part 3)—A Stoichiometry Calculator. You will submit the work you complete in this lesson to your teacher for feedback, and you will have the opportunity to revise and add to it in other lessons. Remember to keep a copy of your Stoichiometry Calculator in your Chemistry 20 course folder so you can revise it as you work through this module.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.17. Page 2
Module 6—Stoichiometry
 Explore
Explore
 Read
Read

© wrangler/shutterstock

© Dutchy, Oisterwijk/iStockphoto
People often don’t think about it, but chemical reactions involving gases are quite common. Do you recall the ideal gas law? This law was an important relationship that equated the number of moles of a gas to the conditions at which it is contained (pressure, volume, and temperature).
Read “7.3 Gas Stoichiometry” on pages 294 to 296 of your textbook. Work through “SAMPLE problem 7.3” and “COMMUNICATION examples” 1 and 2.
Use your notes or your textbook to write the following relationships that you will refer to many times in this lesson:
-
ideal gas law
-
volume of a gas at standard temperature and pressure (STP)
-
volume of a gas at standard ambient temperature and pressure (SATP)
 Discuss
Discuss
Are the general steps for stoichiometry calculations the same for both solids and gases? Do you agree with the statements and explanation provided in the paragraph following “COMMUNICATION example” 1 on page 295 of your textbook? Would it be possible to summarize the methods used to complete stoichiometry calculations for both solids and gases? Are the “SUMMARY” and the “Stoichiometry Calculations” in the margin on page 296 of your textbook adequate?
Post your answers to these questions to the discussion board. Read the postings from two other students. Send your answer to your teacher.
 Self-Check
Self-Check
Complete “Practice” questions 1 to 3 on page 296 of your textbook.
 Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
Use the following lab activity to complete the first part of the Module 6: Lesson 3 Assignment that you saved to your course folder earlier in this lesson. Save the copy of Lesson 3 Assignment containing your answers to your course folder. You will submit this assignment to your teacher later in the lesson.
1.18. Lab
Module 6—Stoichiometry
 Lab: Producing Hydrogen
Lab: Producing Hydrogen
Purpose
In Lesson 2 you tested predictions that were made using the stoichiometric method. In this lab activity you will perform another experiment to test whether predictions made using the stoichiometric method are valid for gases.
To prepare for this investigation, read “INVESTIGATION 7.3: Producing Hydrogen” on pages 305 and 306 of your textbook. Construct a data table in the space provided in your Module 6: Lesson 3 Assignment. As you read through the procedure, record your observations in the table.
Problem
Are predictions made using the stoichiometric method supported by experimental evidence?
Procedure
View a virtual presentation of this investigation, Producing Hydrogen.
Analysis
Answer the Analysis questions in your Module 6: Lesson 3 Assignment.
1.19. Page 4
Module 6—Stoichiometry
 Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
The Case Study questions are to be answered in your Module 6: Lesson 3 Assignment. Open your saved copy of the assignment now.
 Case Study: Producing Hydrogen for Fuel Cells
Case Study: Producing Hydrogen for Fuel Cells
Throughout this module you have considered the use of hydrogen and oxygen as reactants in the main engines of the Space Shuttle. Hydrogen and oxygen gases can also be used in fuel cells—devices that convert chemical potential energy into electrical energy. Hydrogen-oxygen fuel cells have been an essential component of the space program. The fuel cells have produced electricity to run the electronic components within spacecrafts since the 1960s. Many questions exist concerning possible sources of hydrogen for fuel cells. The lack of clear answers to these questions has limited the large scale use of these devices.
Read “Case Study: Producing Hydrogen for Fuel Cells” on pages 297 and 298 of your textbook. Then answer “Case Study Questions” 1 to 3 on page 298. Record your answers in your Module 6: Lesson 3 Assignment. Save the copy of Lesson 3 Assignment that contains your answers to this case study and the lab to your course folder. Also, submit a copy of your Module 6: Lesson 3 Assignment to your teacher.
1.20. Page 5
Module 6—Stoichiometry
 Reflect and Connect
Reflect and Connect
 Module Assessment (Part 3)—Stoichiometry Calculator
Module Assessment (Part 3)—Stoichiometry Calculator
In Lesson 2 you modified your automatic Stoichiometry Calculator to calculate the mass for substances involved in a chemical reaction. Retrieve the latest version from your course folder. Modify your Stoichiometry Calculator and instructions to use and predict either the pressure or volume of a gaseous substance involved in a reaction.
Submit an electronic version of your Stoichiometry Calculator and revised instructions to your teacher, along with a brief explanation of how you constructed the spreadsheet. Provide an example of how you tested the spreadsheet to ensure that it works properly.
As in the previous lesson, you will be allowed to modify your Stoichiometry Calculator using the feedback you get from your teacher. Remember to keep a copy of your Stoichiometry Calculator in your course folder so you can revise it as you work through this module.
 Reflect on the Big Picture
Reflect on the Big Picture

© Blaz Kure/shutterstock
© Michael D Brown/shutterstock
Further development of fuel-cell technology and processes to produce hydrogen may mean that cars that use fuel cells may become more common in the future. In 2007, hydrogen-powered vehicles—due in part to the stoichiometry of the energy-producing reaction—had a limited driving range. In addition, few locations existed for refilling hydrogen-powered vehicles.
 Assessment
Assessment
Submit the following assessment items to your teacher:
-
Module 6: Lesson 3 Assignment
-
Discuss
-
Module Assessment (Part 3)—Stoichiometry Calculator
1.21. Page 6
Module 6—Stoichiometry
 Lesson Summary
Lesson Summary
In this lesson you explored the following essential question:
-
How is the stoichiometric method applied to reactions that involve gases?
You learned to apply your knowledge of stoichiometry to predict the quantities of gaseous substances involved in chemical reactions. You also completed a laboratory experiment where you confirmed the data you collected was consistent with the predictions you made. You then saw examples of how knowledge of the stoichiometry of gases is important to the operation of many technologies, including combustion processes and hydrogen-oxygen fuel cells.
In the next lesson you will look at the stoichiometry of reactions that involve solutions.
1.22. Lesson 4 Intro
Module 6—Stoichiometry
Lesson 4—Solution Stoichiometry
 Get Focused
Get Focused

Image courtesy of NASA
The ability to launch humans into space and then support them while they are there involves the coordination of many systems. It might surprise you that circuit boards like the one pictured below are involved in all systems of a spacecraft. Whether the system is designed to control the flow of fuel into a rocket engine or to control the composition of gases within the crew compartment of the spacecraft, electronic circuits are involved.
©vadim kozlovsky/shutterstock/11241025
The manufacture of a circuit board actually involves removing the copper metal from a plate. Proper treatment and handling of the industrial waste from this process attempts to reclaim the copper. The process involves knowledge of the stoichiometry of aqueous solutions.
In the previous lessons you learned to use stoichiometry to predict quantitative changes of solid and gaseous substances. As you were shown in Modules 4 and 5, many chemical processes occur in aqueous solutions. Knowledge of stoichiometry involving solutions is essential in understanding many industrial and environmental issues.
In this lesson you will use stoichiometry to predict the quantities of substances in chemical reactions that involve solutions.
Essential Questions
-
How is the stoichiometric method applied to reactions that involve solutions?
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
Save a copy of the Module 6: Lesson 4 Assignment to your course folder. You will receive more information about how to complete this assignment later in this lesson.
In Lesson 4 you will continue to work on the Module Assessment (Part 4)—Stoichiometry Calculator. You will submit the work you complete in this lesson to your teacher for marking. Before you submit the final version of your Stoichiometry Calculator, you may wish to check the scoring guide and other information listed in the Module Assessment section. Remember to keep a copy of your Stoichiometry Calculator in your course folder.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.23. Page 2
Module 6—Stoichiometry
 Explore
Explore
 Read
Read

© Elemental Imaging/shutterstock
As you learned in Module 5, quantitative aspects of a solution include the concentration of solute and volume of solution. Can you recall a relationship that exists between a solution’s concentration, its volume, and the number of moles of solute it contains? What might be similar about how you would use this relationship and how you have used other relationships, such as ![]() and PV = nRT, during your study in this module?
and PV = nRT, during your study in this module?
Read “7.4 Solution Stoichiometry” on pages 300 to 302 of your textbook. Work through “SAMPLE problem 7.4” and the “COMMUNICATION example.”
 Discuss
Discuss
Look at the “Stoichiometry Calculations” in the margin on page 302 of your textbook. Construct a flow chart that outlines the steps involved in stoichiometry calculations. The flow chart should summarize the use of the formulas you have used to perform calculations regardless of whether the known or desired substance in a reaction is a solid, gas, or part of a solution.
Post your flow chart to the discussion board for your class. Read and comment on the postings from two other students. Revise your flow chart using the comments relayed to you from your classmates. Place a copy of your finished product in your course folder and submit a copy to your teacher.
 Self-Check
Self-Check
Complete “Practice” questions 1 to 3 on page 302 of your textbook.
 Self-Check Answers
Self-Check Answers
“Practice” questions 1 to 3, page 302
H2SO4(aq)
+
2 NH3(aq)
→
(NH4)2SO4(aq)
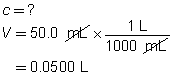
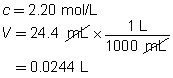
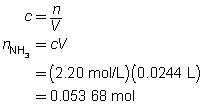
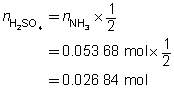
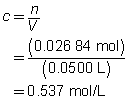
The concentration of the sulfuric acid solution is 0.537 mol/L.
3 Ca(OH)2(aq)
+
Al2SO4(aq)
→
3 CaSO4(s)
+
2 Al(OH)3(s)
c = 0.0250 mol/L
V = ?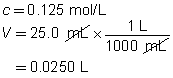
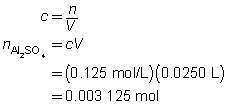
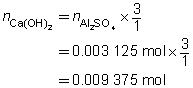
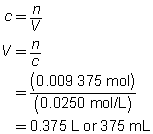
The volume of the calcium hydroxide solution required is 375 mL.
2 FeCl3(aq)
+
3 Na2CO3(aq)
→
6 NaCl(aq)
+
Fe2(CO3)3(s)
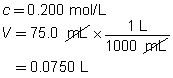
c = 0.250 mol/L
V = ?
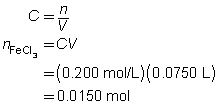
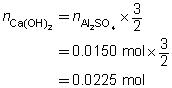
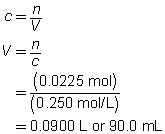
The volume of the sodium carbonate solution required is 90.0 mL.
1.24. Lab
Module 6—Stoichiometry
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
Use the following lab activity to complete the first part of the Lesson 4 Assignment that you saved to your course folder earlier in this lesson. Save the copy the assignment containing your answers to your course folder. This completed assignment will be submitted to your teacher later in the lesson.
 Lab: Analysis of Silver Nitrate
Lab: Analysis of Silver Nitrate
View the lab activity, Lab Analysis of Silver Nitrate
1.25. Page 4
Module 6—Stoichiometry
 Reflect and Connect
Reflect and Connect
 Module Assessment (Part 4)—Stoichiometry Calculator
Module Assessment (Part 4)—Stoichiometry Calculator
Throughout this module you have constructed and modified a Stoichiometry Calculator that allows you to calculate automatically the quantity of a substance involved in a chemical reaction. Retrieve the latest version from your course folder. Modify your Stoichiometry Calculator to use data pertaining to one solution to predict either the concentration or the volume of another solution involved in a chemical reaction.
Submit an electronic version of your Stoichiometry Calculator and revised instructions to your teacher, along with a brief explanation of how you constructed the spreadsheet.
This is your last opportunity to test and perfect your Stoichiometry Calculator to ensure that it works properly and that the instructions you provide are clear and easy to follow. You may wish to refer to the scoring criteria in the Module Assessment section to ensure that your effort will receive as high a grade as possible.
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
Retrieve the copy of the Lesson 4 Assignment you saved to your course folder earlier. Complete all of the questions. You may wish to use the Stoichiometry Calculator you developed to check your work as you complete the assignment. Save your work to your course folder.
 Reflect on the Big Picture
Reflect on the Big Picture
As you have seen throughout this module, launching and operating the Space Shuttle relies on the knowledge of stoichiometry. Can you think of an example where knowledge of solution stoichiometry could be applied?
 Assessment
Assessment
Submit the following items to your teacher:
-
Module 6: Lesson 4 Assignment
-
Discuss
-
Module Assessment (Part 4)—Stoichiometry Calculator
1.26. Page 5
Module 6—Stoichiometry
 Lesson Summary
Lesson Summary
In this lesson you explored the following essential question:
-
How is the stoichiometric method applied to reactions that involve solutions?
You learned to perform calculations using values for concentration and volume of aqueous solutions involved in chemical reactions. Your ability to complete stoichiometric calculations for aqueous solutions can be combined with the skills you learned for solids in Lesson 2 and gases in Lesson 3.
Your study in this lesson has also allowed you to see situations where solution stoichiometry is used to finish materials, make new substances, and monitor and test solutions.
1.27. Module Summary/Assessment
Module 6—Stoichiometry
Module Summary and Assessment
 Module Summary
Module Summary
Stoichiometry is a method of predicting quantitative relationships within chemical systems. Knowing the quantity of substances involved in a process is important when planning or analyzing a process that will involve a chemical change.
Think back to the questions you were asked at the beginning of the module:
-
How do scientists and engineers use mathematics when analyzing chemical change?
-
How is a balanced chemical equation used to predict quantities of species involved in a chemical process?
In this module you viewed a balanced chemical equation as a recipe for a process, where coefficients of the species involved describe the amounts of particles involved. You also used your knowledge of mathematical relationships to calculate the quantity of matter participating in a process from measurable data—including mass, concentration, volume, and pressure. Finally, you verified the predictions made using the stoichiometric method by collecting and analyzing evidence obtained through laboratory investigations.
 Module Assessment
Module Assessment
The assessment in this module consists of the following:
-
Assignments for Lessons 1, 2, 3, and 4
-
Module Assessment (Parts 1, 2, and 3)—Stoichiometry Calculator submitted to your teacher for review and feedback
-
Module Assessment (Part 4)—Stoichiometry Calculator submitted to your teacher for a final grade
Scoring Guide for the Stoichiometry Calculator
Score |
Criteria |
5 |
The design and function of the product is clearly explained. Operation of the Stoichiometry Calculator is straightforward and results in immediate, correct answers for all types of systems (mass, gases, and solutions). Correct and logical algorithms are used.
|
4 |
The design and function of the product is well explained. Operation of the Stoichiometry Calculator is straightforward and results in immediate, correct answers for all types of systems (mass, gases, and solutions). Correct and logical algorithms are used.
|
3 |
The design and function of the product is explained. Operation of the Stoichiometry Calculator results in immediate, correct answers for all systems (mass, gases, and solutions). Correct and logical algorithms are used.
|
2 |
The design and function of the product is poorly explained. Operation of the Stoichiometry Calculator involves more than simple input of a few values. The use of the calculator does not consistently result in correct answers for all systems (mass, gases, and solutions). Generally correct algorithms are used for most systems.
|
1 |
The design and function of the product has one or more serious flaws. The use of the calculator does not consistently result in correct answers for any systems, but evidence of an attempt is provided.
|
0 |
The response is not appropriate for a 20-level student. |
1.28. Module Glossary
Module 6—Stoichiometry
Module Glossary
gravimetric analysis: the determination of masses of substances
law of conservation of mass: a law stating that in any physical or chemical system, the initial mass of the system will be identical to the final mass of the system
mole ratio: a mathematical statement of the proportion of each substance involved in a chemical process relative to one another
net ionic equation: a type of balanced chemical equation that lists only the reacting particles
stoichiometry: a method of predicting or analyzing the quantities of the reactants and products participating in a chemical process
yield of a reaction: a measured quantity of product obtained by a chemical reaction, often expressed as a percentage of maximum yield