Week 1 - Fluids in Technological Devices
| Site: | MoodleHUB.ca 🍁 |
| Course: | Science 8 LearnNet |
| Book: | Week 1 - Fluids in Technological Devices |
| Printed by: | Guest user |
| Date: | Monday, 17 November 2025, 5:54 PM |
Description
Week 1 - Fluids in Technological Devices
1. Unit Introduction and Particle Theory of Matter
Unit Introduction and Particle Theory of Matter
|



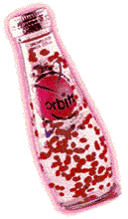
Orbitz is a soft drink that was made by Clearly Canadian in which gel like beads seem to be suspended in the drink. When the bottle is opened the beads seem to be made of water and the whole drink simply pours like water.
Why do the beads float in the fluid and not sink? If the beads pour like water when opened, then why do they not mix with the fluid when they are bumped or moved?
Clearly we need to learn more about the nature of fluids!
| An Orbitz Investigation:
The Net Result By Tim Graham
The beverage industry says that the average American drinks one gallon (3.8 L) of soft drinks each week. Considering that a 1995 census estimated the U.S. population to be 263,874,000 people, that translates into about one billion liters a week! This huge volume means a potentially large profit for soft drink manufacturers, who constantly try to entice you to change brands. One such manufacturer is Clearly Canadian Beverage Corporation, headquartered in Vancouver, British Columbia. Clearly Canadian recently released a unique product they hope will gain a portion of the U.S. marketplace. Sold under the product name Orbitz, this "out-of-this- world" noncarbonated fruit-flavored soft drink is sure to get your attention. Floating in the clear liquid are colorful "orbs," gelatin-like spheres containing complementary flavors that float about in the beverage, seeming to defy gravity. It is this unique property that the manufacturer is counting on to grab the consumers' attention. Half the fun of Orbitz is playing with it before you drink it. No amount of twisting, turning, shaking, heating, cooling, etc., seems to affect its "gravity-defying" properties. But what keeps these flavor spheres suspended? There must be more to Orbitz than meets the eye. Defying GravityOn the basis of a first observation, it would seem that the phenomenon of the floating orbs is density related. If the colored spheres were to remain suspended in the beverage medium, then the spheres and the beverage should have nearly identical densities. Experimentation showed that the density of the orbs and the surrounding liquid are indeed nearly identical. Both have a density between 1.030 and 1.035 g/mL. But one would expect that a two-component system, even of almost identical densities, would separate during the days when the product was being transported from the manufacturing facility to the store shelf. Eventually, all the orbs should sink to the bottom or float to the top, but this never happens to Orbitz. The colored spheres seem to flaunt the law of gravity! Are the flavored spheres held back by something we can't see? The search for an answer was on. Our investigation continued with a simple check of the ingredients listed on the back of the Orbitz bottle. Most are common ingredients found in many brands of soft drinks: water, high-fructose corn syrup, sugar, natural flavors, citric acid, sodium citrate, colors. But a couple are unusual. What are "xanthan and gellan gums"? Could these hold the answer to Orbitz's gravity-defying property? Xanthan gum is a polysaccharide (many-sugar) gum produced by the bacterium Xanthomonas campestris. The primary chemical structure of this gum consists of a cellulose backbone with trisaccharide (three-sugar) side chains and repeating pentasaccharide (five-sugar) units. This giant polymer (its molecular weight exceeds 106 amu [atomic mass units]) has been used in processed foods for years as a stabilizer and an emulsifying agent. (An emulsifying agent is a chemical agent that helps keep one substance evenly dispersed in another. In mayonnaise, egg yolk is the emulsifying agent that keeps the oil droplets dispersed in water. Otherwise, these two "unlike" chemicals would not mix.) Although xanthan gum is a free-flowing powder that dissolves readily in water, just a small amount will make the solution highly viscous. Orbitz is certainly not a highly viscous (viscosity is defined as "resistance to flow") solution, therefore it seemed unlikely that xanthan gum held the answers to our questions. If there were to be a "chemical" answer to the question of how the colored gel spheres were suspended in the Orbitz beverage, it seemed as though gellan gum was the last hope. Gellan gum is a recent addition to the ingredients lists of many processed foods. This polysaccharide is made by Pseudomonas elodea, a bacterium common to the lily pond. According to food-processing experts, gellan gum provides many advantages over more traditional food additives. A gellan gum solution acts like a "gel" that holds particles in suspension but, unlike other gelling agents, it does not significantly increase the solution's viscosity. Chemical engineers incorporated this "Fluid-Gel" technology into the manufacturing of Orbitz. The gellan gum provides a support matrix, something like a microscopic spider web, that holds the flavored spheres in position. An additional benefit of using gellan gum is that it has a visual clarity approaching that of water, and adding sugar promotes this clarity even more. It would seem that Orbitz and gellan gum are a match made in soft-drink heaven! So the answer is finally in hand. The colorful gel-like spheres that first catch our attention do not really defy gravity but are being held in place by a spider web-like support system that is invisible under normal viewing conditions. While the manufacturer claims the unique beverage is "out-of- this-world," we might better regard it as a phenomenon of misplaced worlds. The world of consumer marketing has just been invaded by Pseudomonas elodea,a chemically savvy bacterium from the lily pond!
References "From lily pond to the table." Food Processing, March 1995, p 97. |
Lesson 1
Textbook Readings
ScienceFocus 8
Pages 4-12
or
Science in Action 8
Page 33
Before starting this lesson, take a few minutes and list all of things you already know about solids, liquids, and gases. How are they similar? How are they different? In this lesson, you will be introduced to the particle theory of matter and how it is used to explain the properties of fluids.

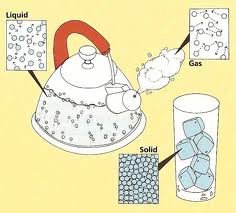
The particle theory of matter is one example of a scientific model . Scientific models help scientists to picture, in an imaginative way, processes in nature that cannot simply or directly be seen. For example, in the particle theory of matter, the individual particles would be far too small and fast-moving to be observed directly. The molecules in the diagram below represent water molecules and how they behave when heated.
|
Particle Theory of Matter
· all matter is composed of tiny particles |
The word fluid comes from the Latin word meaning "to flow." The two most common fluids are liquids and gases. Gases and liquids are fluids because they have no fixed shape and they can flow. The following chart compares liquids and gases and describes why they are considered to be fluids
 |
 |
|
Liquid
|
Gas
|
| have a definite volume | do not have a definite volume |
| when gravity is present, a liquid takes the shape of its container | evenly distributed throughout the surface of any object that they rest upon |
| particles in a liquid state are much further apart than those in a solid | particles in a gaseous state are much farther apart from neighboring particles compared to the particles in a liquid |
| molecules in a liquid state are free to move around within the liquid | gaseous particles move about freely and completely fill their container |
| the particles are closer together than those in a gas | the particles are further apart than those in a liquid |
|
Experiment Time: Investigating Particles
 Scientists use the particle theory of matter to explain the properties of solids, liquids, and gases. Problem: What are the properties of solids, liquids, and gases? Materials: - small block of wood - 2 litre plastic pop bottle - 2 litre measuring bowl (cup) - water - 2 balloons - small pieces of paper Procedure: 1. Prepare a chart similar to the one shown below.
  6. Pour the water from the pop bottle into the measuring bowl. Does the water retain the shape of the pop bottle or does it assume the shape of the measuring bowl? Record your observations in the Properties column of your chart. 7. When you poured the water from one container to another, did the appearance or the amount of water change? Record your observations in the Properties column of your chart.  9. Change the shape of the balloon by squeezing and twisting the balloon. What happens to the air inside the balloon? Record your observations in the Properties column of your chart. 10. Inflate the second balloon but do not tie it. Place the end of the balloon near the tiny pieces of paper and release the balloon. Describe what happens to the small pieces of paper when the balloon is released. Record your observations in the Properties column of your chart. |
2. Fluids
Fluids
Textbook Readings
ScienceFocus 8
Pages 4-12
or
Science in Action 8
Page 13
Background Information Fluids are found just about everywhere you look. You will find them in your home, in technological devices (machines), and in nature.
|
Experiment Time: Identifying Fluids in Your Home
 Problem: How many fluids are in your home? Materials: - access to your refrigerator - cupboard with cleaning supplies - other areas of the house  
Procedure: 1. Prepare a chart similar to the one shown below.
|
|
Experiment Time: Fluids in Nature
 Another key function of fluids is to support life. In this activity, you will investigate the role of fluids in nature.
Materials: - pictures provided - access to electronic and print resources Procedure: 1. Prepare a chart similar to the one shown below.
|
|
© 2002 Alberta Online Consortium
|
3. Uses of Fluids
Uses of Fluids
Textbook Readings
ScienceFocus 8
Pages 4 to 12
or
Science in Action 8
Pages 14 to 17
In Lesson 2, you identified the properties of fluids. In Lesson 3, you will investigate some ways that we use fluids.
Background Information Fluids are important because they make it easier to transport, process, and use different materials even if these materials are solids. The following activities will give you a better understanding of the importance of fluids by investigating slurries , lubricants , the manufacturing of an aluminum pop can, and ketchup. A slurry is a mixture of water and solids. The water is used to transport the solids over a long distance. Slurries have been used in the gold mining industry for more than a hundred years.
|
Experiment Time: Panning for Gold
 Obtain some fine copper beebee pellets or small ball-bearings from your local hardware store. Mix 50 mL of the "gold" with approximately 2 litres of course sand. Place the mixture into a bucket and add enough water to make a slurry. Use an old pie pan to scoop up a bowl of slurry and swirl it over another bucket or large pot. Do not tip the pan too far and add plain water while swirling until only the "gold" pellets remain in the bowl. Explain how a slurry makes it easier to separate the gold from the sand? |

|
Experiment Time: Bake a Cake
 Make sure you have your parent's permission before starting this activity. Obtain a commercial cake mix from the grocery store in your area. Follow the instructions on the package and bake the cake. As you are enjoying the results of your investigation, make notes on the importance of fluids in the process.  |
4. Properties of Fluids
Properties of Fluids
In Lesson 3, you discovered that fluids are important because they make it easier to transport, process, and use different materials even if these materials are solids. In Lesson 4, you will investigate the properties of fluids that make them useful in technological devices.
Background Information
Fluids are very important in the operation of many machines. Air, for example, is a fluid that is used in the tires of a car or bicycle. The hydraulic system in bulldozers is used to lift heavy loads. The brake system in most vehicles uses fluids to transmit pressure from the driver's foot to the brake pads inside the wheels of a car. In an automobile, engine oil not only to lubricates, but this fluid cools hot engine parts, keeps the engine free of rust and deposits, and seals the rings and valves against leakage of hot gases. In this lesson, you will explore the properties of fluids that are used in machines.
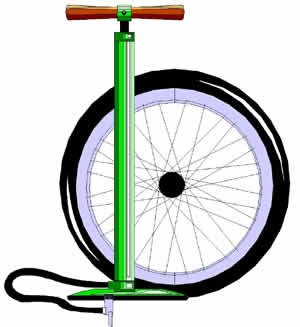
Inflating Tires

You may have had the opportunity to use a small pump to add air to the tires of a bicycle. Why does your tire inflate? What are the properties of gas? A gas is composed of identical molecules that behave like very small, elastic spheres. The elastic spheres are spaced relatively far apart and are in constant motion but can be compressed into a smaller space.
Regardless of the size or shape of the containing vessel, a gas will completely fill it. The molecules of a gas are constantly striking against each other and against the walls of the tire. Gases, because of their ability to completely fill containers, exert pressure on all sides of a container. Compressing the gas into a smaller area increases the pressure in the container. Because the air is compressed into a smaller area, there are more collisions between the particles and the temperature of the system will increase.
Hydraulic Systems
The basic scientific principle behind a hydraulic system is that particles in a liquid cannot be compressed as much as those in a gas. A force applied at one point can be transmitted to another point using an incompressible fluid. The fluid that is usually used is some type of oil. The bulldozer can lift a much heavier load because the force is usually multiplied in the process.

Reducing Friction
Click on the picture to play the clip.
Lubricants are an integral part of all engines and most machinery. The basic functions of a lubricant are to reduce friction, heat removal, and suspend contaminants. The viscosity of an oil is the property that determines its ability to flow. A quick-flowing oil (one of low viscosity) deposits a thin film on the internal parts of an engine, whereas a slow flowing oil (one of higher viscosity) deposits a thicker film.
|
Experiment Time: A Simple Hydraulic System
 It may not be possible to complete this activity unless you have access to the materials suggested below. Problem: Can you make a model of a simple hydraulic system? Materials: - 2 syringes (may be able to obtain the syringes from your local pharmacy) - 30 cm of rubber tubing ( must be the same size as the openings on the syringes) - sink - water What To Do 1. Fill a sink about half-full of water. 2. Place the two syringes and rubber tubing into the water and try to get the air out of the tubing. 3. Fill the syringes about half full with water and then connect the water filled tubing to both syringes. 4. Empty the sink but conduct the rest of the activity in this area to reduce the mess that you might make. 5. Squeeze the plunger on one of the syringes and observe what happens to the plunger on the other syringe. Reverse the process and repeat several times. |
|
Experiment Time: Reducing Friction
 Problem: Can a lubricant reduce friction? Materials: - 5 mL of cooking oil or hand lotion - towel Precautions If you use cooking oil, make sure to wear old clothing and conduct this activity away from your computer. Procedure: 1. Press one of your hands on to the surface of your desk or table. Rub your hand over the surface as fast as you can for several seconds. Describe the process and the temperature of your hand. Record your observations. 2. Place 5 mL of cooking oil or hand lotion on to the surface of your desk or table. Rub your hand over the lubricated surface as fast as you can for several seconds. Describe the process and the temperature of your hand compared to the first trial. Was it easier or harder to move your hand over the desk this time? Was your hand warmer or cooler when you used the lubricant? Record your observations. |
Exercise 1.4: Inflating a Tire
6. Unit 1 Section 1 Quiz
Unit 1 Section 1 Quiz
You will have two opportunities to write this section quiz. This quiz consists of 10 questions. Use the results from your 1st attempt to help you prepare for your second attempt. Your best score will be taken as your assessment mark. You have 10 minutes to complete this multiple choice quiz.