Week 3 - Properties of Gases and Liquids Explained by the Particle Model
| Site: | MoodleHUB.ca 🍁 |
| Course: | Science 8 LearnNet |
| Book: | Week 3 - Properties of Gases and Liquids Explained by the Particle Model |
| Printed by: | Guest user |
| Date: | Monday, 17 November 2025, 5:49 PM |
Description
Week 3 - Properties of Gases and Liquids Explained by the Particle Model
1. Measuring Viscosity Using Flow Rate
Measuring Viscosity Using Flow Rate
Textbook Readings
ScienceFocus 8
Page 40
or
Science in Action 8
Page 40
|
Viscosity is a liquids internal resistance or friction that keeps it from flowing.
|
Background Information There are many ways to measure viscosity. The following demonstration tests flow rate of various substances.
Exercise 3.1
|
Experiment Time: Viscosity Lab
(Set up this lab physically if you would like)

(click assignment icon below to get tables for observation and analysis then click on the lab) |
2. Changing Viscosity
Changing Viscosity
Textbook Readings
ScienceFocus 8
Pages 45, 46, 48, 49
or
Science in Action 8
Pages 39, 41
Viscosity is important in many other industries as well and the ability to change the viscosity of a fluid is very important.
View the following multimeda presentation on viscosity in beekeeping.
After reading the section below ask yourself the following question.
What two things effect the rate of flow of honey?

Viscosity and Honey
The viscosity or sticky nature of honey causes it to resist movement through the extractor, pump, pipes, and strainers.
Viscosity describes the rate of flow of a liquid. A thick, slow, flowing liquid has a high viscosity (good body) and a thin liquid has low viscosity.
Liquid honey with a high moisture content has a low viscosity. Honey with a low moisture content has high viscosity. Cold honey has a high viscosity, warm honey a low viscosity. Therefore, a low moisture, cold honey is hard to handle.
The viscosity of honey can be lowered temporarily by applying heat, but as the honey cools viscosity increases again. Heating honey lowers viscosity (increases rate of flow), making easier the jobs of extracting, pumping, straining, and settling. Warm weather also helps lower viscosity. Therefore, the more honey extracted when weather is warm, the better.
Heating honey, unfortunately, releases the aromatic volatile oils, destroys enzymes and darkens the honey. The problem is one of moving the honey as quickly as possible with the least possible damage to its quality.
Viscosity and Temperature
"As slow as Molasses in January." Why is molasses in January so slow? Why is it slower in January then June or July? Because viscosity is dependent on temperature .
There are several different methods of measuring the rate of flow.
| Concept Review You may drop an object into a liquid as in the previous activity or You may pour the liquid through a narrow opening like a funnel and measure the time it takes to flow through. |
One option is to heat it up . Many restaurant serve syrup warm so it will be easier to pour.
Another method is to mix it with another substance. Water has a relatively low viscosity (meaning it is not thick), therefore as long as the substance will mix with water , adding water can lower the viscosity.
Mixing fluids to change viscosity Since water has a relatively low viscosity then mixing it with something that has a relatively high viscosity should reduce the overall viscosity.
This is true as long as the two substances mix. For example water could be mixed with molasses to thin it but it could not be used to reduce the viscosity of oil because oil and water do not mix.
Exercise 3.2: Flow Rate Lab

|
|
Enrichment:
Optional Research / Discussion topic: In which other industries is viscosity important. |
|
© 2002 Alberta Online Consortium
|
3. Viscosity Review
Viscosity Review

Take a minute and think about that strange drink again.
Could viscosity be why the beads do not mix with the liquid? Perhaps the beads are more viscous then the surrounding water?
Exercise 3.3 - Viscosity Mystery
|
© 2002 Alberta Online Consortium
|
4. Density Introduction
Density Introduction
Textbook Readings
ScienceFocus 8
Page 52 and review figures 129 and 130
or
Science in Action 8
Page 42
Background Information
Density is a term which is similar to the word crowded . A large number of people in a small space is a high density of people where as a small number of people in the same space is a small density.
Which picture has the greatest density of people? 

The same applies to molecules. A large number of molecules in a space means a large density and a small number of molecules in the same space means less density.
Which of the following substances has the largest density ? 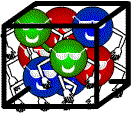

Density is related to the idea of crowded and it is also related to the idea of weight . Logically a substance with lots of molecules (more dense) is heavier then a substance with fewer molecules (less dense). Scientists do not like to use weight because it is not an accurate measurement, instead they prefer the term mass . They both measure how heavy an object is but mass is a more accurate measurement then weight. Different substances have different densities. The density of water at room temperature is 1.00 g/ml. ( g/ml = grams per milliliter). Generally, substances which are more dense then water sink , substances which are less dense then water float.
Exercise 3.4 Density Exploration (no assignment submission)
Click on the Lab and complete the assessment questions at the bottom of the lab and check your answers.
password: 9418
6. Unit 1 Section 3 Quiz
Unit 1 Section 3 Quiz
You will have two opportunities to write this section quiz. This quiz consists of 10 questions. Use the results from your 1st attempt to help you prepare for your second attempt. Your best score will be taken as your assessment mark. You have 10 minutes to complete this multiple choice quiz. Remember to do this quiz under testing conditions without texts, aids, or help.
Click the icon to go to the quiz.



