Week 4 - Density and Buoyancy
| Site: | MoodleHUB.ca 🍁 |
| Course: | Science 8 LearnNet |
| Book: | Week 4 - Density and Buoyancy |
| Printed by: | Guest user |
| Date: | Monday, 17 November 2025, 5:42 PM |
Description
Week 4 - Density and Buoyancy
Calculating Density
Textbook Readings
ScienceFocus 8
Pages 53 & 57
or
Science in Action 8
Page 43 and study the activity on pages 44, 45
http://learn.argyll.epsb.ca/Science/Sci8/AOC/

Background Information
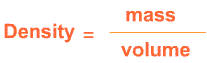 |
The mass of an object divided by the volume of that object is the density .
|
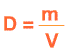 |
|
Experiment Time: Interactive Density Lab 1
 Make sure you are in the Interactive Physics Module then
Click on Virtual Density Lab
|
|
Experiment Time: Density Lab 2
 Try logging into learnalberta.ca first and then come back and click on this icon.
LA13
9418
Click on double box arrow (top left) to print out the lesson worksheets
If the above icon doesn't work try this link: http://learnalberta.com/Search.aspx?lang=en&search=density&grade=&subject=
Then click on Density Laboratory located as the second link on this search using the word "density."
|
Changing Density
Density and Temperature Can the density of an object change? To answer a question with a question, does ice float? Of course ice floats and therefore it must be less dense then liquid water yet they both are made from water. Therefore the density of water changes with temperature.
As a general rule (ice is an exception) warmer substances are less dense. As you know, when substances are heated, the molecules move more rapidly. As the molecules move more rapidly, they take up more space. Less molecules in a given area means lower density.
Density and Concentration The relationship between density and concentration is simple: The more solute (i.e. salt or sugar) the more dense.
It is very difficult to swim in the dead sea of Israel because there is a large concentration of salt in the water, therefore the water is more dense then man and man floats on the surface of the water.

|
Self-Assessment: Density Review
Review Links:Try the following online density review quizzes. |
|
© 2002 Alberta Online Consortium
|
Buoyancy - Sink or Float
Textbook Readings
ScienceFocus 8
Pages 64 & 65
or
Science in Action 8
Pages 50 & 51
Once back on dry land, your body feels heavy.
Buoyancy is the force in the water that makes you feel light when you are in the water.
Background Information
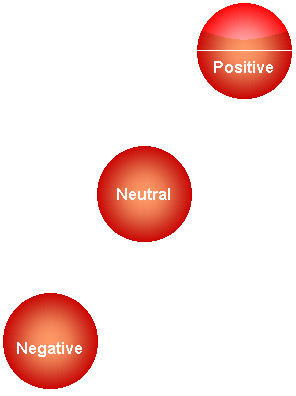
Some objects, when placed in water, float, while others sink, and still others neither float nor sink. This is a function of buoyancy. We call objects that float, positively buoyant . Objects that sink are called negatively buoyant . We refer to object that neither float nor sink as neutrally buoyant .
There are three types of buoyancy
positive buoyancy -objects with positive buoyancy float
neutral buoyancy - neutral buoyancy objects neither float nor sink - they are suspended in the middle
Negative buoyancy - objects with negative buoyancy sink
id: arygllbp
The idea of buoyancy was summed up by Archimedes, a Greek mathematician, in what is known as Archimedes Principle: Any object, wholly or partly immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. From this principle, we can see that whether an object floats or sinks, is based on not only its weight, but also the amount of water it displaces . That is why a very heavy ocean liner can float. It displaces a large amount of water.
 Exercise 4.3: Create a Boat
Exercise 4.3: Create a Boat
|
Think about it!
 Are the globs in this picture are an example of ....
|
|
Enrichment (optional)
Try the Online Buoyancy Review Quiz Click Here to Read about Buoyancy. Use the link at the bottom of the page to try out the buoyancy brain teasers. (Try all three questions at the bottom) |
|
© 2002 Alberta Online Consortium
|
Measuring Buoyancy
Lesson 16: Measuring Buoyancy
Textbook Readings
ScienceFocus 8
Page 67
or
Science in Action 8
No reading

http://learn.argyll.epsb.ca/Science/Sci8/AOC/
Testers like the one shown to the left are often used to test antifreeze levels in vehicles. The rubber hose is inserted into the radiator and when the bulb is squeezed and released, antifreeze is drawn through the hose into the plastic chamber. The coloured balls inside the chamber have different densities. By counting the balls that float, the level of protection from freezing can be determined. Pure water has a density of 1.00 g/mL. Vehicles must have a more dense liquid in their cooling systems to protect them from freezing during cold Alberta winter nights.

Buoyant forces are dependent on density. A hydrometer is a device the measures the density of a liquid. Pure water has a density of 1.00 g/mL.
|
g/mL is "grams per milliliter"
|
Background Information The greater the density of the liquid it is placed in, the higher it will float. It floats higher in more dense liquids and lower in less dense liquids. Try your hand at reading the hydrometer.
Exercise 4.4: Building a Hydrometer
Unit 1 Section 4 Quiz
Our regular quiz is moved to week 5 to include both week 4-5. You can write it after completing week 5.