Week 5 - Fluid Technologies
| Site: | MoodleHUB.ca 🍁 |
| Course: | Science 8 LearnNet |
| Book: | Week 5 - Fluid Technologies |
| Printed by: | Guest user |
| Date: | Monday, 17 November 2025, 5:41 PM |
Description
Week 5 - Fluid Technologies Part 2
1. Pressure
Textbook Readings
ScienceFocus 8
Pages 71, 73-75
or
Science in Action 8
Pages 53, 56-59

Background Information
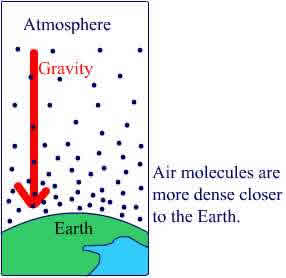
| Air Pressure Like everything on earth, the air in our atmosphere is being pulled down by gravity. Air pressure is strongest close to the Earth.
More molecules = more weight = more pressure
Air pressure decreases as you move further away from the Earth, therefore air pressure changes with altitude. If you go for a ride on an airplane or dive in the mountains, you ears "pop" as your body adjusts to the change in pressure. As air is heated, the molecules move farther apart and the air in the balloon becomes less dense. The balloon floats up in the air. |
 |
 |
As air is heated, the molecules move farther apart and the air in the balloon becomes less dense. The balloon floats up in the air. As the air in the balloon cools. The molecules move closer together and the balloon becomes more dense. The balloon returns to Earth. |
|
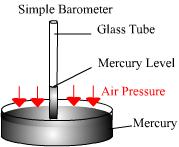
Barometer
Air pressure is measured using a barometer. A simple barometer consists of a thin glass tube in mercury. As the air pressure
|
 |
|
Water Pressure
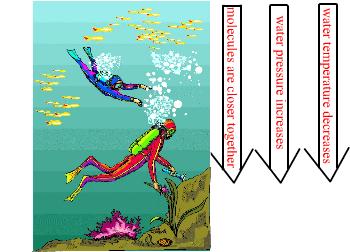
Water pressure also changes as you move downward. As a diver moves deeper in the water the pressure increases because the molecules are packed closer together. Water is not compressible, as shown above, but it is under increasing pressure as the depth increases. Since the pressure is already evenly distributed, because the molecules are already close together, the temperature of the water doesn't increase as its depth increases. Another reason the temperature doesn't increase is because it is further from the source of heat (the sun). There are locations in the ocean, however, that are hotter the lower you travel but that is because those areas are close to a different heat source. These heat sources are vents from cracks in the Earth's crust where magma is closer to the surface of the crust in those locations. |
 |
2. Solubility of Materials in Water
Solubility of Materials in Water
Textbook Readings
ScienceFocus 8
Pages 39-41
or
Science in Action 8
Pages 66-68

Let's explore how solubility can be used to make our lives better.

How do we get salt? Where does it come from? And what processes are used to get it to our table? Let's find out.
The basic forms of salt mining are:
- rock salt mining
- solution salt mining.
- a. Solar evaporation
- b. Vacuum evaporation Use the following web sites to compare and contrast these two mining processes by answering the question below. mechanical production
- solar evaporation
|
|
© 2002 Alberta Online Consortium
|
3. Viscosity Review
Viscosity Review
Textbook Readings
ScienceFocus 8
Pages 39 - 41
or
Science in Action 8
Pages 66 - 68
 |
"You're as slow a molasses in January! "
|
 |
A few lessons ago you learned what that statement means. If you have heard it spoken to you, it was probably by your parents when you didn't want to do something - like clean your room. You learned in the previous lesson that the seasonal temperature changes affect the flow of fluids including molasses.
In this lesson we are going to investigate various technologies that depend on fluids to have fairly specific viscosities. For example, how would you like your ketchup to run like water?
Background Information
Viscosity is an internal property of a fluid that offers resistance to flow. For example, pushing a spoon with a small force moves it easily through a bowl of water, but the same force moves mashed potatoes very slowly. In fact, one of the major differences between styles of mashed potatoes is the viscosity of the starchy mass: some people like their potatoes running and teeming with milk and butter (they are fans of low-viscosity potatoes), while others like their potatoes drier and stickier, so they almost crack rather than flow (these people are devoted to high-viscosity potatoes).
http://www.spacegrant.hawaii.edu/class_acts/ViscosityTe.html
| Let's go back for a moment and review the factors that affect viscosity. 1. Viscosity can be defined as the amount of friction or internal resistance a fluid has. 2. If it has a high viscosity it will be thick and flow very slowly. 3. Thin runny fluids are said to have a low viscosity. 4. So what do we know of that affects the viscosity of a fluid? Temperature! That means if a industry can control the temperature of a fluid it can generally control the viscosity of that fluid. For example, in order easily mold steel it must be heated so that it has a low viscosity and can be poured into molds. |
 |
|
The Alberta Tar Sands
 |
Alberta Oil Sands
(See attached file: Alberta Oil Sands Fact Sheet.pdf) http://www.oilsands.alberta.ca/FactSheets/The_Facts_Aug2011.pdf (See attached file: Oil Sands 101) http://www.imperialoil.com/Canada-English/operations_sands_glance_101.aspx |
|
Experiment Time: Motor Oil
 
|
|
© 2002 Alberta Online Consortium
|
4. Fluid Transport
Fluid Transport
No Textbook
Readings
for this lesson
Have you ever wondered where the natural gas that heats your home comes from? Or why most people in the hospital are connected to bags hanging from poles?
In this lesson we are going to examine how liquids are transported from one place to another. First, we will examine how natural gas is transported across Canada. Second, we will look at the technology that is used in hospitals to give ailing people food, water and medicine.

Background Information
| Intravenous Delivery
Have you ever watched any of those emergency medicine shows on TV? It seems one of the first things they when a person is really sick or injured is to put in an IV ( intravenous ) line. Why? Let's find out. Here is a list of questions that the interviewer in the video is going to ask the nurse at the hospital. After the video, record the answers in the space provided. |
 |
![]() Exercise 5.4A: Health Care
Exercise 5.4A: Health Care
![]() Exercise 5.4B: Natural Gas
Exercise 5.4B: Natural Gas
 Use the document below to find the answers to the following questions. Transportation of natural gas: |
5. Unit 1 Section 4-5 Quiz
You will have two opportunities to write this section quiz. This quiz consists of 10 questions. Use the results from your 1st attempt to help you prepare for your second attempt. Your best score will be taken as your assessment mark. You have 20 minutes to complete this multiple choice quiz.




