Weeks 27 & 28 - Humans Depend on Water Supply and Quality
| Site: | MoodleHUB.ca 🍁 |
| Course: | Science 8 LearnNet |
| Book: | Weeks 27 & 28 - Humans Depend on Water Supply and Quality |
| Printed by: | Guest user |
| Date: | Monday, 17 November 2025, 5:54 PM |
Description
Weeks 27 & 28 - Humans Depend on Water Supply and Quality
Introduction

A
|

B
|

C
|
 D
|
 E |
 
F
|
 
G
|
 H
|
All are linked to water. Let's take a look .
Photograph (a). These sandstone forms were caused by the erosion of ocean waves and tides.
Photograph (b). The scratches in these rocks were caused by the erosion of moving masses of frozen water called glaciers.
Photograph (c). The Saskatchewan Glacier is an example of a river of ice. The glacier not only erodes the land but melting glaciers release large volumes of fresh water into river systems around the world.
Photograph (d). Sea lions are mammals adapted to life in the salt water of the world's oceans.
Photograph (e). Sea stars and many other invertebrates live in the nutrient rich intertidal zone. The intertidal zone is the boundary between the ocean and the land.
Photograph (f) This fish and insect larva are adapted to life in fresh water ecosystems.
Photograph (g) This tanker is spilling millions of litres of crude oil into the ocean. Birds, fish, mammals, and invertebrates will be killed by this toxic mixture.
Photograph
In this unit, you will explore the distribution of fresh water and salt water ecosystems on the Earth. You will describe the processes of erosion and deposition resulting from wave action and water flow. You will investigate the distribution and health of living organisms in aquatic environments. Finally, you will analyze human impacts on aquatic ecosystems.
|
© 2002 Alberta Online Consortium
|
Lesson 1

Seen from space you can tell that Earth is a water planet. Without this water, life as we know it wouldn't exist. The most extra ordinary thing about this water is that large amounts of it are in liquid form - a very rare thing in our universe.
In this lesson we are going to examine the distribution and characteristics of this water. We will first get an overview of the world's water and then look closer to home to see how water is distributed in Alberta.
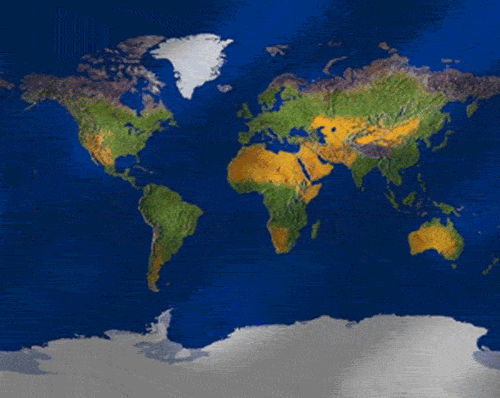
Let's examine the Earth from an alien's point of view. Upon arriving in orbit you begin to examine the Earth's features. What can we see about the Earth from this vantage point?
|
Watch this video to get some inspiration about "using less" of our Earth's resources. |
© 2002 Alberta Online Consortium
|
|||||||||||||||
Lesson 2

| Have you ever made Kool-Aid? You might have noticed that as you stirred the liquid, the sugar seemed to disappear as it dissolved into the water. You have made a solution. The water was the solvent - that part in the greater amount. The sugar is called the solute. Can you think of any other solutes in the Kool-Aid? |
 Your favorite pop is water with dissolved chemical flavoring, sugar and carbon dioxide gas. |
 |
Water is called the universal solvent because it is the most common solvent in the world. It can't dissolve petroleum based materials like gas and oil, but other inorganic materials - even some rocks can be dissolved in water! How many things can you think of that can be dissolved in water?
|
|
Other materials besides solids can dissolve in water. Dissolved gasses such as oxygen, nitrogen and carbon dioxide can be found in most water bodies like lakes, rivers and oceans. |
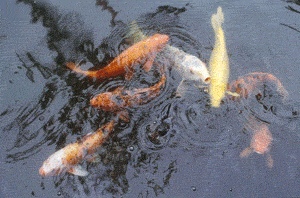
Animals that live in the water use the dissolved oxygen in the same way we use the gaseous oxygen found in the air - for cellular respiration
|
| Carbon dioxide is another gas commonly found in water. When it combines with water it forms carbonic acid. This weak acid can slowly dissolve limestone and other rocks. This cave was formed as water slowly dissolved the under ground limestone over hundreds of thousands of years. Some of those dissolved minerals remain in the water. Would you like to know more? |
 |
Exercise 1.2: Make a Naked Egg
Water - Hard and Soft
All natural bodies of water contain dissolved minerals and gasses. Chemicals such as iron, calcium, magnesium and lime can be dissolved in water. When they are found in large amounts, generally greater than 200 mg / litre, it is called hard water.
|
Why is hard water a problem? 1. Lime scale can block hot water heaters, pipes, kettles, dishwashers, shower heads, clothes washers, etc. 2. Scum can build up on bathtubs, sinks, toilets and shower walls. 3. It can leave spots on your dishes and glasses. 4. The water may be discoloured or have a bad taste. 5. Soap does not lather (foam up) as well. 6. Clothing does not feel soft and doesn't get as clean as it should. 7. Hair feels tangled and frizzy. 8. Skin can dry out and conditions like eczema may worsen. |
|
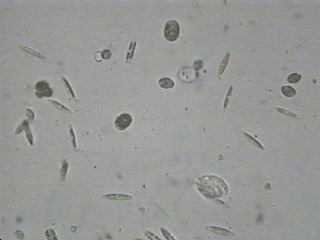
There is much more in a lake, pond or river than dissolved minerals and gasses. As you know from the information you learned in module 2, 'Cells and Systems', most water bodies contain life. For example, algae can be one celled and microscopic but if its present in the water in the billions it will turn the water green in colour. Other forms of life may not be easily seen, but they all have a great impact on the quality of the water. Have you ever wondered where fish go to the bathroom?
|
But You Can't Drink Any Of It - Yet!
 |
One of the great pleasures in life is to hike in the Rocky Mountains. But even here, you shouldn't drink the water in those beautiful mountain streams without treating it first.
 A protozoan parasite called 'Beaver Fever' has contaminated almost all the lakes, rivers and streams in the Rocky Mountain watershed. Symptoms are also described on this website. If you are camping and want to use water from a lake or stream, it's important to ensure that it is clean and safe. Read this website to learn some different methods of purifying water. |
From Fresh Water?
 Many sailors ship wrecked at sea have died of thirst. Do you know why? The water is too salty to drink. Those who do drink it become even more thirsty as their body tries to get rid of the excess salt. SALINITY AND ITS VARIABILITY... Oceanographers report salinity (total salt content) and the concentrations of individual chemical constituents in sea water -- chloride, sodium, or magnesium for example -- in parts per thousand, for which the symbol o/oo is used. That is, a salinity of 35 o/oo means 35 pounds of salt per 1,000 pounds of sea water. Similarly, a sodium concentration of 10 o/oo means 10 pounds of sodium per 1,000 pounds of water. The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, snowfall, wind, wave motion, and ocean currents that cause horizontal and vertical mixing of the saltwater. This page is very detailed (maybe a bit too much!) but it does explain salinity very well. http://www.palomar.edu/oceanography/salty_ocean.htm |
Sea water contains hundreds of dissolved atoms and molecules, including magnesium, calcium, bromine, chlorine, carbonate, sulfur, iron, silicate, and dissolved gases such as oxygen, nitrogen, and carbon dioxide. These, and many more chemicals combine to make the seas and oceans salty. salinity In oceanography, the salinity of seawater is a measure of the amount of total dissolved salts expressed as the number of grams of dissolved material in one kilogram of seawater. It is more strictly defined as the weight in grams of the dissolved inorganic matter in 1 kg of seawater after all bromide and iodide have been replaced by the equivalent amount of chloride, and all carbonate converted to oxide. In practice it is determined from conductivity and temperature measurements in the laboratory or from conductivity, temperature and pressure measurements in situ via the use of a CTD. |
Lesson 3
Read pages 339 - 344

What is Potable Water?
Potable water is water that is safe to drink. But how do we judge if water is safe or not? We know by now that there could be dissolved chemicals and microscopic life in the a glass of water that we wouldn't be able to detect with our normal senses. In this lesson we will learn how water is tested and judged to be safe to drink.
|
Every major city has a water treatment facility. They treat drinking water to make it potable and treat wastewater before releasing it into reservoirs. Edmonton's Gold Bar Wastewater Treatment Plant is an award winning facility! |
 |
These are some of the items that drinking water is tested for:
|
| Treating Fresh Water So Its Fit to Drink Most communities in Alberta have water treatment plants that test and purify the water for their communities. People on farms can have their well water tested by provincial or private labs to ensure that it is safe. This is particularly important in farming country as chemicals from fertilizer like nitrites, and organisms such as bacteria from animal wastes can leach into the ground water and cause severe illness and death. This happened in a small farming community in Ontario a few years ago. Methods of Treatment Water treatment plants use filters and chemical treatment to ensure that our water is safe. At certain times of the year, when the water from lakes and rivers is dirty from runoff or has high amounts of algae, treatment plants add more chlorine to the water to ensure that the water remains safe. Many water treatment plants are changing over from chlorine to ozone to kill bacteria and other microorganisms.
|
|
© 2002 Alberta Online Consortium
|
Read pages 344 - 345
Lesson 4
Its all very well to talk about the need to clean fresh water to ensure its safety, but what if you really don't have access to enough fresh water to start with? For example, there are many desert countries that just don't have enough water to go around. But many of these same countries lie along an ocean or sea. In this lesson we will examine three methods of transforming salt water to fresh water.
|
Did You Know?
A Canadian scientist has developed a system for catching fog on the desert coast of Chile. This fresh water is then used by local villagers. In the past they had to rely on expensive truck loads of water. |
 |
|
The Atacama Desert in Northern Chile is so dry that there are places where rain has NEVER been recorded. Other parts of the desert may go for decades without feeling a single drop of rain. The location is the reason that no rain falls on the Atacama. The Andes block any moisture containing clouds from the east, and the cold waters of the offshore Humboldt current cause any rain on the west side of the Andes to fall at sea. Clouds do form over the area, and a cover of fog, known locally as the camanchaca, often flows inland from offshore. However, the camanchaca and the stratocumulus clouds lack enough moisture to produce rainfall. The small fishing village of Caleta Chungungo has developed an innovative way of harvesting the moisture from the camanchaca. The system consists of large nets arrayed across the slopes perpendicular to the landward flow of the camanchaca. The fog condenses on the mesh and the water drips into a trough. The collected water flows from the trough down through a pipe to a reservoir. |
|
Desalination is the process of removing the salt from seawater to produce fresh water. Read on to learn more about it: (retrieved from National Geographic Magazine, "The Big Idea": March 15, 2010. http://ngm.nationalgeographic.com/big-idea/09/desalination)

Three hundred million people now get their water from the sea or from brackish groundwater that is too salty to drink. That’s double the number a decade ago. Desalination took off in the 1970's in the Middle East and has since spread to 150 countries. Within the next six years new desalination plants may add as much as 13 billion gallons a day to the global water supply, the equivalent of another Colorado River. The reason for the boom is simple: As populations grow and agriculture and industry expand, fresh water—especially clean fresh water—is getting scarcer. “The thing about water is, you gotta have it,” says Tom Pankratz, editor of the Water Desalination Report, a trade publication. “Desalination is not a cheap way to get water, but sometimes it’s the only way there is.”
And it’s much cheaper than it was two decades ago. The first desalination method—and still the most common, especially in oil-rich countries along the Persian Gulf—was brute-force distillation: Heat seawater until it turns to steam, leaving its salt behind, then condense it. The current state of the art, used, for example, at plants that opened recently in Tampa Bay, Florida, and Perth, Australia, is reverse osmosis, in which water is forced through a membrane that catches the salt. Pumping seawater to pressures of more than a thousand pounds per square inch takes less energy than boiling it—but it is still expensive.
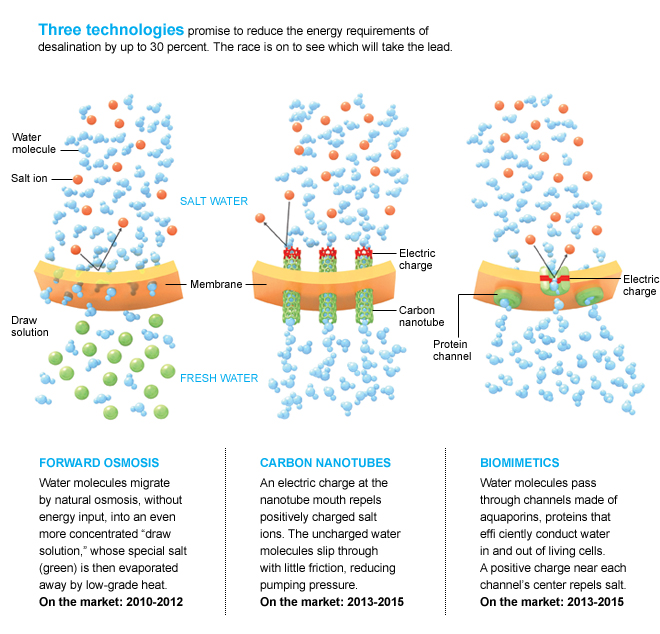
Researchers are now working on at least three new technologies that could cut the energy required even further. The closest to commercialization, called forward osmosis, draws water through the porous membrane into a solution that contains even more salt than seawater, but a kind of salt that is easily evaporated. The other two approaches redesign the membrane itself— one by using carbon nanotubes as the pores, the other by using the same proteins that usher water molecules through the membranes of living cells.
None of the three will be a solution for all the world’s water woes. Desalination inevitably leaves behind a concentrated brine, which can harm the environment and even the water supply itself. Brine discharges are especially tricky to dispose of at inland desalination plants, and they’re also raising the salinity in parts of the shallow Persian Gulf. The saltier the water gets, the more expensive it becomes to desalinate.
What’s more, none of the new technologies seem simple and cheap enough to offer much hope to the world’s poor, says geologist Farouk El-Baz of Boston University. He recently attended a desalination-industry conference looking for ways to bring fresh water to the war-torn Sudanese region of Darfur. “I asked the engineers, ‘What if you are in a tiny village of 3,000, and the water is a hundred feet underground and laden with salt, and there is no electricity?’ ” El-Baz says. “Their mouths just dropped.” —Karen E. Lange
In the process of the distillation of seawater the water undergoes a change of state. First, the seawater is heated. This evaporates the water but leaves the salts behind. The water vapor is then cooled and as it condenses, it returns to the liquid state as fresh water! Read about the scientific principles that explain distillation.
|
You may recall that osmosis is the movement of water molecules through a semi-permeable membrane. The water will move from an area of high water concentration through the membrane to an area of lower water concentration. The flow will stop when both sides are equal in concentration. In reverse osmosis, the water molecules will flow against the concentration gradient and move from an area of lower water concentration through the membrane to an area of higher concentration. How is this done? Read this fact sheet about a desalination project in Adelaide, Australia. |
 These and other marine mammals can drink sea water because they can get rid of the excess salt in their bodies. But humans must find ways of turning saltwater to fresh water or perish.
|
Did You Know?  In the past, when sailors set out on a long ocean voyage they had to carry all the fresh water that they would need. Today, modern sailors have portable desalinators so they can make all the fresh water they need. Check out this site if you would like to know more. |
 |
You have two options for your Section 1 Quiz - a multiple choice quiz or a written response quiz. You may choose to write the multiple choice or the written response. You only need to write one of them, so the choice is up to you! You are allowed to try both quizzes if you want, but it is not required.
The quiz attempt with the highest grade will be the one that is recorded on your report card.
The multiple choice quiz has 10 multiple choice, matching, and true/false questions. You have 15 minutes to complete it. As soon as the quiz submitted it will be auto-graded and you will receive a grade immediately.
Click the image above to start your quiz
The written response quiz has 5 short answer questions. Your responses must give a complete and detailed answer to the question. You have 20 minutes to complete the quiz. This quiz needs to be manually marked by your teacher, so you may have to wait a few days to get feedback and a grade.
Click the image above to start your quiz