Lesson 1: Elements and Atoms
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 BOOTSTRAP EXAMPLE |
| Book: | Lesson 1: Elements and Atoms |
| Printed by: | Guest user |
| Date: | Sunday, 7 December 2025, 4:42 PM |
1. Introduction
Science and Technology in Society
During World War II, an insecticide called DDT was used to prevent insects from transmitting disease. An unforeseen effect of using DDT was the large decline in raptor populations, such as the peregrine falcon, due to a weakening of their eggshells. DDT was banned in most developed nations in the 1970s and 1980s, and raptor populations are now recovering. This example demonstrates the importance of doing a risk-benefit analysis when considering the use of technology or a change to processes.
Even today, DDT is still used in many developing nations where malaria continues to be a problem. Malaria annually affects millions of people worldwide. Due to this, the benefit of using DDT to prevent malaria and save lives is given a greater priority than the risk posed to the environment and to sensitive bird populations.
You are encouraged to consider the following important question as you study this unit.
- What is the nature of science and technology, and what is their relationship?
- How should science-related societal, political, economic, ethical and environmental issues be addressed?
2. Science, Technology and Society
Science involves the study of natural products and processes with the purpose of describing, explaining, and predicting natural events. Science is theoretical knowledge, emphasizing ideas and conducting research.
Technology, on the other hand, focuses on the skills, processes, and equipment involved with manufacturing products and performing tasks. Technology is driven by cost, reliability, efficiency, and productivity. There are many cases where technology precedes science. The battery is a good example. Volta is credited with inventing the battery in 1800 but scientists didn't understand the chemical reaction within the battery for another hundred years. In the case of Volta's battery, a new technology revealed the need for new science. In other situations, new technology can enhance existing science. Ex. computer science enhanced scientific researches.
This course is more than the study of theoretical knowledge and empirical knowledge (practical knowledge) about the natural world. A key component throughout this course will be making connections between science, technology, and societal (STS) issues as you learn about chemistry. There are many different perspectives that can be adopted when analyzing an STS connection: economical, ethical, ecological, political, scientific, societal, and technological.
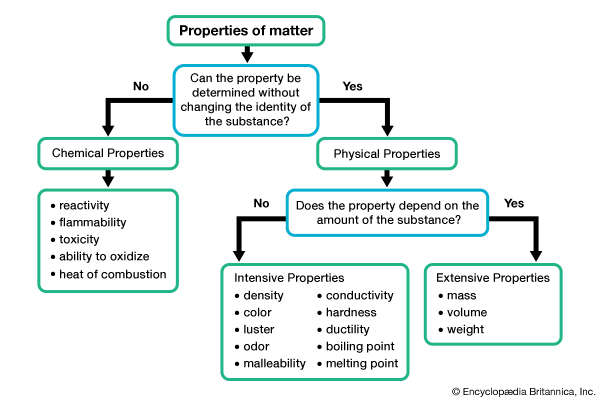
3. Classification of Matter
Watch this short video, and go to textbook pages for details.
4. Periodic Table
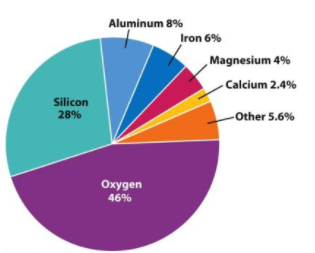
Did you know that Earth's crust is composed mainly of oxygen?
The oxygen in Earth's crust is not in gaseous form as it is in the atmosphere. Instead, oxygen is a component of solid compounds, such as rocks and minerals. Elements can exist in various forms. How does the structure of atoms differ when atoms of the same element have different forms?
In this section, you will review models of the atom, and investigate how these models are used to explain various forms of matter.
The organization of data into patterns or common characteristics helps scientists understand the material they are studying. In dealing with the matter, elements are classified according to their atomic numbers. The atomic number indicates the number of protons found within the atom. Along with the atomic number, the elements are also categorized according to how they react. The result of this classification is the Periodic Table of the Elements. Make sure to try the following activity.
5. Atomic Structure and Ions
Atomic Structure and Ions
Our present knowledge of matter results from the work of numerous people over several centuries. With thoughtful observation, these people built on the discoveries of others to reveal how substances behave. They compared their research and gradually developed more exact methods of testing new ideas. Models or concepts which could explain experimental results and predict future findings often became accepted theories. But with each discovery, these theories were challenged. Often the theories were rejected or greatly modified.
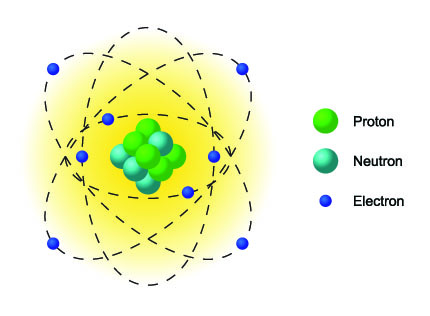
An atom has a specific structure consisting of three parts:
- Proton: symbol p+
Positively charged particle located in the center of an atom which makes up a larger portion of the mass of an atom - Neutron: symbol no
Neutral-charged particle located in the center of the atom. It makes up a large portion of the mass of the atom. - Electron: symbol e-
Negatively charged particle surrounding an atom. It has very little mass and moves about the nucleus in an electron cloud.
Calculating Numbers of Subatomic Particles:
Protons = Atomic Number
Electrons = Atomic Number( in a neutral atom)
Neutrons = Atomic mass - Atomic number
Example: Lithium has atomic number 3 so it has 3 protons and 3 electrons. It has an atomic mass of 6.79. We round this off to the nearest whole number, 7, since we cannot have a part of a proton nor neutron. So, there are 4 neutrons ( 7-3=4).
Isotopes:
Neutrons in some elements may vary. Uranium, for example, may gain or lose neutrons to form isotopes of uranium, each having a different mass.
U235 is a light isotope having 3 fewer neutrons than the most common form of uranium. U239 is a heavy isotope having 1 more neutron than the most common form.
Key Concepts
proton
neutron
electron
isotopes
|
-subatomic particle |
-relative charge |
-relative mass(amu) |
|---|---|---|
| p+ | 1+ | 1836.12 |
| no | 0 | 1838.65 |
| e- | 1- | 1 |
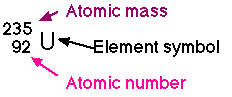
6. Models of Atom
In this course, we will be studying five different models. However, we will be focusing more on the Bohr model and the energy-level diagram. In lesson four, we will revisit the electron cloud model. Study the following two diagrams that demonstrate the same information about phosphorous, then review the models of atom activity.

Electron configurations are as follows for a maximum number of electrons per energy level.
- 1st energy level: maximum of 2 electrons
- 2nd energy level: maximum of 8 electrons
- 3rd and higher energy levels: 8 electrons
The atoms of elements combine to form millions of different compounds with varying physical and chemical properties. This combining atom is called chemical bonding. Chemical bonds involve the electrons in the outermost energy levels of the reacting atoms. All atoms tend to fill their most outer energy level with electrons to become more stable.
For example, hydrogen atoms have a single electron in the first energy level. Helium atoms have 2 electrons that fill the first and outermost energy level. Hydrogen atoms are highly reactive, helium atoms are highly stable.
Atoms do not combine randomly, they react to lose, gain, or share electrons in order to completely fill the outermost energy level. Atoms can fill their outermost energy levels by the transfer of electrons from one atom to another. One atom gains electrons while the other atom loses electrons. Doing so, no longer neutral, atoms become ions. Ions with opposite charges attract each other. This is how some compounds form.
Models of Atoms:
Try the following activity summarizing the models of atoms.