Lesson 3: Chemical Reactions and Equations
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 BOOTSTRAP EXAMPLE |
| Book: | Lesson 3: Chemical Reactions and Equations |
| Printed by: | Guest user |
| Date: | Sunday, 7 December 2025, 4:41 PM |
1. Chemical Reactions and Bonding
Chemical changes take place when molecules or elements interact with other elements or molecules to form new chemical compounds. In order for a reaction to take place between molecules and or atoms, these molecules must come into contact with each other. For example, the reaction betting ammonia with hydrogen chloride to form ammonium chloride shown below.
NH3 +HCL → NH4Cl
This equation does not clearly show what has happened. For these molecules to react, the pair of electrons on nitrogen must collide with the hydrogen atom of the HCl. This collision must not only be precise as to the angle of the collision, but it must also have enough energy to break the bond between the hydrogen atom and the chlorine atom and form a new bond between the hydrogen atom and the nitrogen atom. Energy is released when a bond is formed.
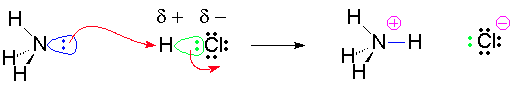
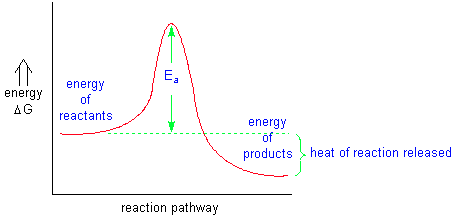
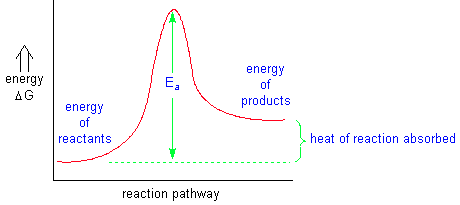
The reaction rate is measured by the change in concentration of one of the reactants or products over a measured period of time. Reaction coordinate diagrams are used to visualize the energy changes in chemical reactions. Some initial energy must be applied to any reaction in order to get the reaction started. This energy is called the energy of activation(Ea).
If a reaction releases more energy than it takes to keep it going, it is called an exothermic reaction. If a reaction requires a constant application of energy to keep it going, it is called an endothermic reaction.
A catalyst is something that, when added to a chemical reaction, will increase the reaction rate without undergoing a permanent change. Although it appears that only Ea is lowered for a catalyzed reaction, the actual reaction pathway must change due to the involvement of the catalyst with the reactants. The energy released for the reaction remains the same. Catalysts are used extensively in biochemical reactions in order to decrease the energy demands for the animal or plant.
The law of conservation mass states that matter can neither be gained nor lost in a chemical reaction. The number and type of atoms in the reactants must exactly equal the number and types of atoms in the products. The arrangement of the atoms will be different because new compounds are formed.
Key Concepts
Chemical reactions
Balancing chemical reactions
Chemical amount

Learn more: study Textbook reading p. 46-63
2. Reaction Types
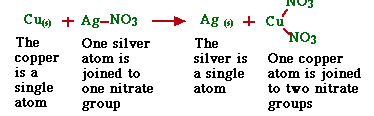
1.Single Replacement
In these reactions, one of the elements in the compound is replaced by another element.
Cu(s) +AgNO3(aq)--> Ag(s) +Cu(NO3)2(aq)
2. Double Replacement
In these reactions, both the metal and nonmetal will be replaced between two compounds.
Mg(OH)2(s) + HCl(aq)--> MgCl2(aq) +HOH(l)

3. Synthesis Reaction
A synthesis reaction, in the simplest sense, involves elements as reagents and the formation of a compound (a substance composed of more than one element) as the product, often as the only product.
Fe + S ---> FeS
Iron + Sulfur = Ion sulfide
4. Decomposition
Decomposition reactions are chemical reactions in which chemical species break up into simpler parts.
E.g. 2NH3 → N2 + 3H2
Compounds don't need to break down into elements in a decomposition reaction.
E.g.
(NH4)2CO3(s) →2NH3(g)+CO2(g) +H2O(g)
5. Hydrocarbon Combustion
When hydrocarbons such as gasoline, methane, and sucrose are burned (combusted), they always produce energy, carbon dioxide gas, and water vapor. The act of burning is actually just a rapid reaction with oxygen.
E.g. methane gas is burned (combusted).
CH4(g) + 2O2(g) →CO2(g) + 2H2O(g) +Energy
A Final Note...
There are other categories of chemical reactions. The two broadest categories are the acid-base reactions and oxidation-reduction reactions.
You will learn more about the former in the following units, and the latter in Chemistry 30.
3. Balancing Chemical Reactions
A chemical equation is a way to describe what goes on in a chemical reaction, the actual change in a material. Chemical equations are written with the symbols of materials to include elements, ionic or covalent compounds, aqueous solutions, ions, or particles. The following diagram shows the components of a chemical reaction.
![]()
Suppose you are camping and have been sitting around a fire all night. You have burned an awful lot of wood. You get up the next morning only to find that there are ashes left. Where did all the wood go? This seems to contradict the law of conservation of mass. In reality, most of the mass has been given off as water vapor and carbon dioxide. If you could collect the water vapor, carbon dioxide, heat, and all the ash, you would find that it would equal the same mass as the wood you burned. This law ties in well with the atomic theory of matter. According to the theory, atoms are never created or destroyed. In chemical reactions, the atoms and molecules are simply rearranged. Molecules may be broken apart and new ones may be formed, but the atoms in the products are the same ones that were in the reactants.
Often you will see the descriptions of the materials in the reaction in parentheses after the material. Gas is shown by (g). Solid material is shown by (s). Liquid is shown by (l). A material dissolved in water (an aqueous solution) is shown by (aq). This when balancing chemical reactions comes to play. Now, let's see how we can balance reactions with a few examples.
Example 1. Mg(s) + O2(g) → MgO(s)
The formula, MgO, indicates that for every magnesium atom there is an oxygen atom. We start out with one magnesium atom and two oxygen atoms, ending up with one magnesium atom and one oxygen atom. What happens to the other oxygen? It must be accounted for!
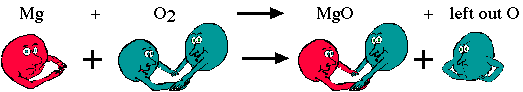
When the oxygen molecules break apart to react with the magnesium atom, the lone oxygen is free to bond. We assume that the only elements present are those listed. This means that oxygen can bond with another magnesium. This will make all of the atoms more stable.
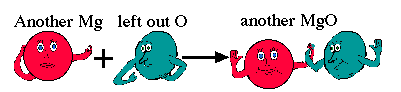
In other words, we have now used up two magnesium atoms and created two units of magnesium oxide. We show this by placing numbers in front of the molecule.
2Mg(s) + O2(g) → 2MgO(s)
This is a balanced equation. It can be read as follows: Two atoms of magnesium react with one molecule of oxygen to form two molecules of magnesium oxide.
The goal is to ensure that the same number of the same type of atoms appear on each side of the equation. To balance an equation you may start with any atom but you may find it helpful to start with the one present in higher numbers.
Example 2. S8(s) + Li → Li2S(s)
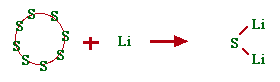 Start with the sulfur atoms. 8 on the reactant side means we can make 8 units of Li2S(s). We must place an 8 in front of the Li2S. The number of lithium atoms required to make 8 units of Li2S(s) is 16. We must then have 16 on the reactant side. The equation becomes:
Start with the sulfur atoms. 8 on the reactant side means we can make 8 units of Li2S(s). We must place an 8 in front of the Li2S. The number of lithium atoms required to make 8 units of Li2S(s) is 16. We must then have 16 on the reactant side. The equation becomes:
S8(s) + 16Li → 8Li2S(s)
These two examples lead us to the following three steps when balancing chemical equations:
Steps For Balancing Chemical Reactions:
1. Write a word equation for the reaction
2. Write the correct formula for the reactants and products.
3. Determine coefficients that make the equation balanced.
Example 3. H2O → H2(g) + O2(g)
You can, of course, balance your reactions, using the method you want, but I would like to suggest a method for you to use is the Balance (B) & Count (C) Method.
Let's balance the above reaction using this method.
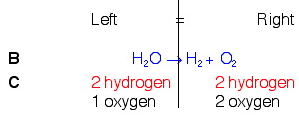
So, looking back at our rules, knowing that we can only add coefficients at the beginning of compounds, and knowing that we need a total of 2 oxygen on the left, we will put a 2 in front of water on the left. Once we do this, we will count again and see if we need to do anything else.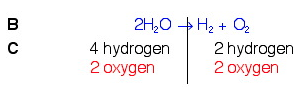
In balancing the oxygens, we changed the number of hydrogens on the left. This is OK. But we still need to balance them. To do this, we will put a 2 in front of the hydrogen count on the right. This should fix the problem.
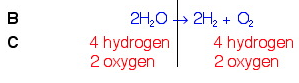
We now see that the equation is balanced.
To sum up, the Law of Conservation of Mass states that in a chemical reaction there is no loss of mass. Each type of element will have the same amount before the reaction and after the reaction, or as reactant and product. But you can't change the materials that participate in the reaction, so you must write an integer coefficient in front of each material in the reaction to ensure every type of atom has the same number on each side of the reaction.
4. Chemical Amount: the Mole
The mole is a unit representing a certain number of things. Much the same as a dozen or a gross, but instead of being 12 or 144 things, a mole is 6.02 x 1023 things.
It is sometimes referred to as Avogadro's number and it is defined as the number of atoms in exactly 12 grams of Carbon-12. The mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is assigned a unit called atomic mass units (amu). In order to make more sensible, we use amu as gram/ mol.

The mathematical relationship between mol, molar mass and mass can be expressed as
![]()
n: mol
m: mass (gram)
M: molar mass (gram/mol)
Using the periodic table, we can calculate the molar mass (mass of 1 mol) of the H2O molecule.
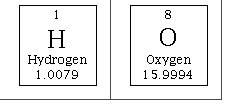
| 2H= 2 mol x 1.01 g/mol | = 2.02 g/mol |
|---|---|
| 1 O= 1 mol x16. g/mol | = 16.00 g/mol |
| 1 HOH | = 18.02 g/mol |
This brings us to the four steps that help us calculating a substance's molecular weight.
- Determine how many atoms of each different element are in the formula.
- Look up the atomic weight of each element in the periodic table
- Add the results of step three together and round off as necessary.
Calculate the molar mass of CuSO4•5H2O
Solution:
Atomic mass of Cu + S+ 4O + 5(2H +O) = 249.68 g/mol
Example 2:
Calculate the number of mol found in 10.0 g of NaCl.
Solution:
Step 1. Atomic mass of Na= 22.9898, Cl= 35.4527 g/mol (again, found on the periodic table)
Step 2. The molar mass of NaCl= 22.9898+35.4527 g/mol= 58.4425 g/ mol
Step 3. If 58.4425 g of NaCl is 1 mol, then 10.0 g will be x mol.
x= 10g/58.4425 g/mol= 0.1711 mol.
Example 3:
In gram, calculate the mass of 0.50 mol of H2O.
Solution:
Using the formula,
![]()
m= n x M
m= 0.50 mol x 18.0152 g/mol
m= 9.0076 g
5. Self-Check Assessment
SC1. For each word equation, write the chemical formula, balance the reaction, and determine what kind of reaction it is. Then, hit each tap to compare your answer with the given solution.
SC2. Try to answer the following two questions. Then, hit the dropdown sign to check your answer.