Lesson 1. Solutions and Molarity
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 BOOTSTRAP EXAMPLE |
| Book: | Lesson 1. Solutions and Molarity |
| Printed by: | Guest user |
| Date: | Sunday, 7 December 2025, 4:38 PM |
1. Introduction: Properties of Solutions
Solutions are homogenous mixtures of two or more elements or compounds. Pure substances are substances that cannot be separated by physical processes. Elements and compounds are pure substances. Any mixture of pure substances that is the same throughout is a homogeneous solution. Homogenous solutions can be as found as solids dissolved in liquids, liquids dissolved in liquid, gases dissolved in liquids, gasses dissolved in gases, or solids dissolved in solids. The substance that gets dissolved in the mixture is called the solute, the substance that does the dissolving is called the solvent of the solution.
The heterogeneous mixtures are not the same throughout as you can see their components. Ex. tomatoes in a bowl of salad.
Many ionic compounds in solution react easily with each other in double-replacement reactions to form precipitates. Ionic compounds are most soluble in polar solvents, i.e. H2O(l). Polar solvents bind more strongly to the individual solute ions. The solute is effectively pulled apart into individual ions increasing the surface area between reacting particles. These solute ions are now free to react with other ions in the solution. In the case of a double-replacement reaction, the evidence of ta chemical change is seen with the formation of a precipitate.
Key Concepts
Solution, solvent, and solute
Endothermic and Exothermic reaction
Electrolytic solutions
Molar Concentration
Dilution

2. Energy Changes and Electrolytic Solutions
Endothermic vs. Exothermic Reactions
Breaking bonds is always endothermic while forming bonds is always exothermic. There are two major steps when a solute dissolves in water.
- Endothermic process: energy is being absorbed to break the individual ions that formed the crystal lattice within that ionic solid.
- Exothermic process: energy is released from the system as the individual ions now are surrounded by water molecules.
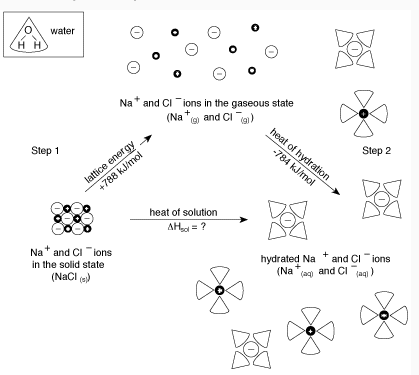
The overall process can be summarized by the following equation:
Final Energy= (Energy to break apart solute) + (Energy to bind solute ions to water)
- Energy absorbed to break apart the solute < the energy released as the ions bind to water. The overall process of dissolving is Exothermic
- Energy absorbed > the energy released. The overall process of dissolving will be endothermic.
Can you tell if the reaction shown from the diagram above endothermic or exothermic?
NaCl(s) + 788 kJ/mol → Na+(g) + Cl-(g) ( Endothermic)
Na+(g) + Cl-(g) → Na+(aq) + Cl-(aq) + 784 kJ/mol (Exothermic)
Since the Net Energy = 788 kJ/mol - 784 kJ/mol= 4 kJ/mol, this reaction is endothermic reaction.
Electrolytic Solutions
Electrolytes are substances that conduct electricity when dissolved in water. Electricity flows from one location to another through a conductive substance that acts as a medium through which electrons can travel.
Metals are often good conductors because they tend to form lattice structures that make it very easy for electrons to bounce from atom to atom in a current. The conductivity of solutions is determined by the concentration of ions dissolved in the solution. The more positivity and negatively charged particles there are in the solution the greater the conductivity will be.
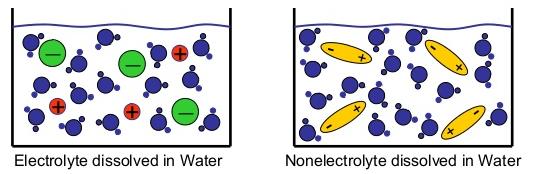
Nonelectrolytes do not conduct electricity when dissolved in water because they are electrically neutral molecules. Ionic compounds are electrolytes. Most molecular compounds are nonelectrolytes, but there are a few exceptions, such as organic acids, and some dissolved gases, like carbon dioxide in an aqueous solution. Acids, bases, and salts form ionic bonds which makes them electrolytes. Substances that form covalent bonds are either nonelectrolytes or poor electrolytes.
3. Molarity
Molarity (molar concentration) is a measure of the concentration of a chemical species in a solution in terms of moles per liter of solution. Molarity can be calculated as 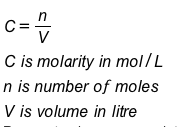
Although molarity is the most common way of expressing concentration, there are other ways as well. However, the units for C, n, and V might change depending on which way is being used. See the example problems below.
Volume-volume percent (% v/v): Vinegar is sold as percent per volume. That is a number of mL per 100 mL of solution. A typical vinegar solution is 5% acetic acid by volume. This means there is 5 mL of acetic acid for every 100 mL of vinegar.
Unit: mL/100mL
Mass-volume percentage (%w/v): Hydrogen peroxide, used for cuts and burns on the skin, is often sold as 3% w/v. This means that there are 3g of solute (hydrogen peroxide) for every 100 mL of solution.
Unit: g/100 mL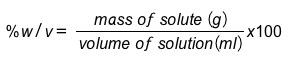
Parts per million (ppm): extremely diluted solutions often state concentration in ppm. The number of mg per L of solution or the number g of solute per 1000 L of solution. There is 2 mg/L of chlorine in pool water.
Units: mg/L or g/kg
Example 1
|
In 130 ml cup of green tea, there is about 120 mg of caffeine. Calculate the concentration of caffeine in this tea as a percentage of weight for volume. |
 |
%w/v = (0.120 g / 130 ml)x100 = 0.09%
Examples 2
A sample of solution contains 47 ppm of lead. What amount of lead is present in 300 mL of H2O(l)
C= 47 mg/L
V= 300 mL= 0.300L
n=?
n= CV = (47 mg/ L)x(0.300L) = 14.1mg ppm.
Example 3
A sample of mouthwash is a 5.0% w/v solution of table salt. What volume can be made from 15g of salt?
C= 5 g/100mL
n= 15.0g
V=?
V= n/ C => 15 g x (5g/100mL) = 300mL
4. Dilution of Solutions
State of Matter
In chemical equation, subscripts are used to indicate the state of matter:
(aq) aqueous
(l) liquid
(s) solid
(g) gas
Example: H2(g) + O2(g) --> H2O(l)
NaCl(s) --> Na+(aq) + Cl-(aq)
A compound that is dissolved in water is no longer in the solid, liquid or gaseous state, The subscript (aq) is written after the chemical formula of a compound is dissolved in water.
Dilution
Most solutions are stored in concentrated form, and diluted for use when required. When an aqueous solution is diluted, the number of moles of solute does not change. Water is added to make the concentration smaller. Since the initial mole (ni) equals the final mole (nf) and n=CV, we can rearrange our formula as:
ni = nf
CiVi = CfVf
Let's see how this equation helps us when solving dilution problems.
Example 1
If concentrated HCl(aq) has a concentration of 12.5 mol/L. What volume of concentrated HCl(aq) will be required to prepare 300 ml of 0.200 mol/ L diluted HCl(aq)?
Solution:
Step 1: Convert 300 ml to L = 0.300 L
Step 2: Calculate the volume of concentrated HCl(aq)
CiVi = CfVf
Vi = CfVf/ Ci
Vi= (0.200 mol/L x 0.300 L) / (12.5 mol/L)
=0.0048 L = 4.8 mL
Preparation of a Dilution
Write out the materials and procedures for preparing the dilution in the following problem.
What volume of a 0.500 mol/L stock solution( original) of NaNO3 is needed to prepare 100 mL of a 0.050 mol/L solution?
Solution:
Step 1: Find the initial volume
CiVi =CfVf
0.500 mol/Lx Vi = 0.100 L x 0.050 mol/L
Vi = 0.010 L = 10.0 mL
Step 2: Materials
NaNO3 solution, beaker, volumetric flask, pipet bulb, wash bottle, funnel, medicine dropper.
Step 3: Procedure:
- Add initial volume to a 100 mL volumetric flask by pipet (in this case Vi=10mL)
- Add distilled water to the volumetric flask and bring the solution to the 100 mL line.
- Insert stopper to the flask, and invert twice or more to mix the solution.


