Click on photos to enlarge.
| step 1: |
Prepare a data table with two columns. Use the names of the two acid solutions listed in the materials as the
headings for your table.
|
| step 2: |
Use the grease pencil or waterproof marker to label one of the test tubes "Ethanoic Acid." Place the test tube into the test tube rack.
|
| step 3: |
Transfer exactly 5.00 mL of ethanoic acid to one of the graduated cylinders. Use an eyedropper to ensure the
meniscus is at the 5.00-mL mark on the cylinder.
 |
Measuring 5 mL of ethanoic acid |
|
| step 4: |
Transfer the ethanoic acid in the graduated cylinder to the “Ethanoic Acid” test tube.
 |
Labelled tubes containing respective acids |
|
| step 5: |
Transfer enough of the ethanoic acid from the test tube to cover the bottom of one of the 50-mL beakers. Use
the conductivity meter to test the solution. Record your observations in your data table. Use the acid in the beaker
to measure the pH. Place one of the strips of pH paper into the remaining solution and record the pH of the
solution in a data table. (If you are using a pH meter, you may have to use a larger volume of acid in the beaker
to cover the electrode at the bottom of the meter. Ensure that you rinse the electrode before testing the pH of
the second solution.)
 |
Ethanoic acid in beaker |
| Results: |
 |
Ethanoic acid undergoing conductivity test |
| |
 |
Ethanoic acid undergoing pH test |
| |
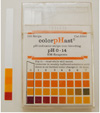 |
pH results of ethanoic acid |
|
| step 6: |
Repeat steps 2 to 5 using the hydrochloric acid solution.
| Results: |
 |
Hydrochloric acid undergoing conductivity test |
| |
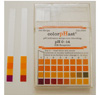 |
pH results (far left) of hydrochloric acid |
|
| step 7: |
Place a strip of magnesium into each of the test tubes. Record the initial response of the magnesium to the
acid and the length of time until a reaction no longer occurs in each of the test tubes. Estimate the amount of
magnesium remaining in each test tube.
| Results: |
 |
Initial response of hydrochloric acid (left) and ethanoic acid (right) |
| |
 |
Response of hydrochloric acid (left) and ethanoic acid (right) after 20 min |
|











