Click on photos to enlarge.
| step 1: |
Use the grease pencil to label a 50-mL beaker
"Acid." Transfer approximately 40 mL of the
acid solution (of unknown concentration) to
this beaker.
|
| step 2: |
Use the grease pencil to label another 50-mL
beaker "Base Sodium Hydroxide." Transfer
approximately 45 mL of the NaOH(aq) to this
beaker.
|
| step 3: |
Attach and secure the burette clamp half-way
along the ring stand.
|
| step 4: |
 |
Remove the burette from the clamp. |
 |
Carefully tilt the burette to wash the inside. |
Carefully wash the burette. Close the stopcock;
then fill the burette one-third full with water from the
wash bottle. Tilt the burette sideways to wash the
inside walls, then tilt it
vertically and hold it over the 250-mL beaker,
which will serve as the waste beaker. Open the
stopcock and transfer the wash water from the
burette into the waste beaker. Repeat this step
one more time.
|
| step 5: |
Place the burette into the burette clamp attached
to the ring stand.
|
| step 6: |
 |
Close the stopcock, place the funnel into the
upper end of the burette, and add approximately
15-20 mL of NaOH(aq) to the burette. Remove
the funnel from the top of the burette. |
|
| step 7: |
Carefully remove the burette from the clamp;
then tilt and wash the inside walls with the
sodium hydroxide solution. Return the burette
to the clamp, position the waste beaker under
the burette, and open the stopcock to drain the
burette.
 |
Remove the burette from the clamp. |
 |
Carefully tilt the burette to wash the inside. |
|
| step 8: |
 |
Close the stopcock, replace the funnel in the
upper end of the burette, and add NaOH(aq) to
fill the burette so that the liquid level is within the
range of 0 to 5 mL. |
|
| step 9: |
Position the burette so that the level of the
NaOH(aq) is at eye level, and measure the
position of the lower surface of the meniscus.
(You should be able to measure this to at least
one decimal place; however, two decimal places
is preferred. You might find that using a card
with a dark line on it improves the visibility of the
bottom of the meniscus.) Record this level in your
table.
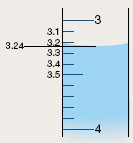
| Results: |
 |
|
|
| step 10: |
Using either a graduated cylinder or a pipette, transfer 10.0 mL of the acid solution to the
125-mL Erlenmeyer flask.
 |
Measuring the acid solution |
 |
Transferring the acid to the flask |
|
| step 11: |
Add 3 to 4 drops of bromothymol blue (or phenolphthalein). Record the colour of the indicator in the acid sample
in your table.

|
| step 12: |
Position the Erlenmeyer flask containing the acid and indicator under the burette; then open the stopcock,
and add a small amount of the solution from the burette to the contents of the flask. As you add solution from
the burette, swirl the contents of the flask to ensure mixing. Repeat, adding small amounts of solution from the
burette to the flask until the indicator changes colour. Record this colour in your table.
 |
Add the first drop of the base. |
 |
Swirl the flask while adding the base. |
| Results: |
 |
The indicator has changed colour. |
|
| step 13: |
Once a permanent colour change has been reached, position the burette so that the level of the NaOH(aq) is at
eye level; then measure the position of the lower surface of the meniscus. Record this level in your table.
| Results: |
 |
|
|
| step 14: |
Transfer the contents of the Erlenmeyer flask to the waste beaker, rinse the flask with some water from the wash
bottle, and repeat steps 8 to 13 to obtain data from three more trials that agree within 0.2 mL.
|
| step 15: |
Disassemble, wash, and return equipment to its proper location. Repeat step 3 to wash the burette.
|













