Module 7—Principles of Chemical Equilibrium
Linking Statements
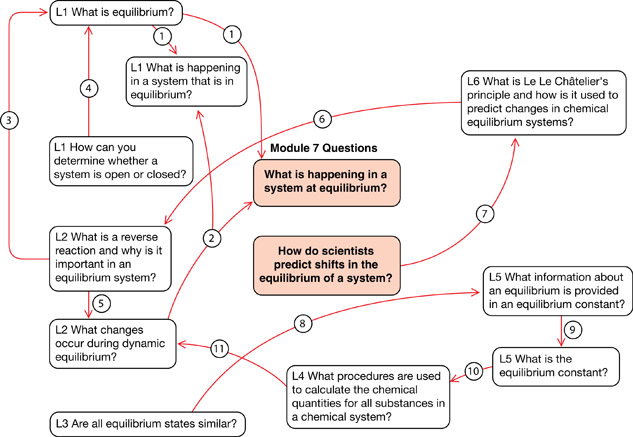
# |
Statement |
1 |
appears to not change—constant set of macroscopic properties |
2 |
equal rates for the forward and reverse reactions |
3 |
and forward reaction define the changes that occur in an equilibrium |
4 |
Is matter or energy being added/removed? |
5 |
reverse reaction describes one of the ways the system can change |
6 |
stress can favour the reverse or forward reaction to move to a new equilibrium |
7 |
using |
8 |
proportions of products relative to reactants is unique to conditions and described using |
9 |
[products]/[reactants] |
10 |
is used to |
11 |
ICE tables used to apply stoichiometry to an equilibrium system |