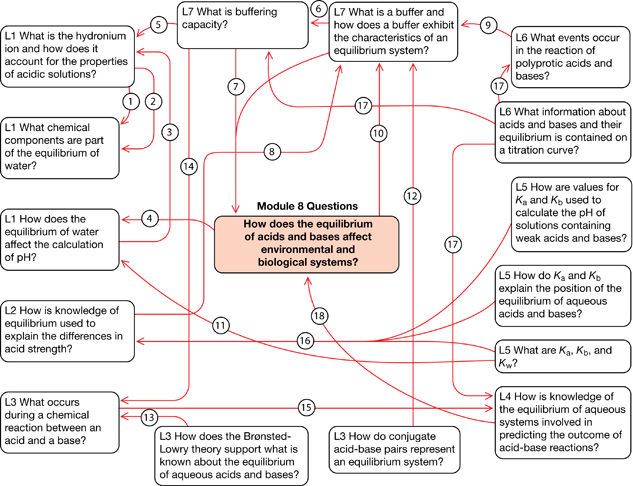
Module 8—Acid-Base Equilibrium
Linking Statements
# |
Statement |
1 |
hydronium is one of the |
2 |
molar concentration of hydronium involved in |
3 |
all acids ionize to produce |
4 |
biological and environmental systems involve |
5 |
ability to neutralize |
6 |
chemical quantities determine the |
7 |
used to protect |
8 |
weak acids and bases used to construct buffer systems |
9 |
many can be used to construct |
10 |
carbonic acid-hydrogen carbonate one example |
11 |
important values for |
12 |
example of buffer components |
13 |
predict reactants in a proton transfer reaction |
14 |
a proton transfer reaction |
15 |
extent of reaction (position of equilibrium) determined by |
16 |
constants describe the |
17 |
pH curve contains this information |
18 |
used to predict possible effects |