Lesson 2. Concentration of Ionic Solutions
1. Ionic Solutions and Dissociation
Dissociation of Ionic Compounds
In the previous lesson, we studied molarity, dilution, and steps in preparing solutions. In these lessons, we will expand on these concepts.
Ionic compounds are created by the mutual attraction of positively charged ions called cations, and negatively charged ions called anions. Structurally, ions differ from atoms as a result of having more or fewer electrons than protons in the orbitals about the nucleus. Having more electrons than protons results in a negative net ionic charge, and a positive net ionic charge is generated when protons outnumber electrons. An ionic compound is electrically conductive as a result of these electron imbalances. Positive and negative ionic charges cause ions from structures through which a current of electrons can flow across all of the positive and negative pathways between ions, particularly when in solution.
Why the ionic solutions are electrolytic while molecular solutions are not?
Study the following two models: 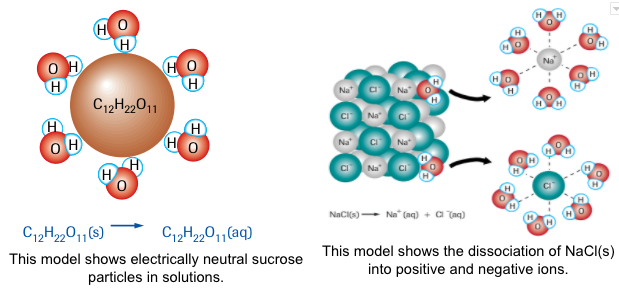
Key Concepts
Dissociation
Concentration of ions
Solubility
Dynamic equilibrium

Learn more go to Textbook p. 192-214
While the first model shows the neutral sucrose molecules surrounded by water molecules, the second model shows that NaCl dissolves and forms positive ions that surrounded by the negative ends of the polar water molecules and the negative ions are surrounded by the positive ends of the polar water molecules. The separation of ions in ionic solution is called dissociation. The equation below shows the dissociation of NaCl;
NaCl(s) --> Na+(aq) + Cl-(aq)
Another example, (NH4)2SO4(s) --> 2NH4+(aq) + SO42-(aq)
Notice that both equations do not show H2O(l) as a reactant in the equation. Water as a solvent is necessary for the process of dissociation. However, it is not consumed and therefore is not a reactant. The presence of water molecules surrounding the ions is indicated by (aq).