Module 7 Intro
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 SS |
| Book: | Module 7 Intro |
| Printed by: | Guest user |
| Date: | Wednesday, 24 December 2025, 10:31 AM |
Description
Created by IMSreader
1. Module 7 Intro
Module 7—Chemical Analysis
Module Introduction
In Module 6 you learned to use the stoichiometric method to predict the quantities of the reactants and products involved in a chemical reaction. In this module you will continue to apply your ability to analyze chemical systems using mathematical relationships.
Is it possible to predict the amount of product that would result if you were given defined quantities of each reactant? You will perform a titration, which is a standard laboratory technique used for chemical analysis. You will then use the results from your titration to communicate information to other students about the substance tested.
Think about the following questions as you complete this module:
- How is stoichiometry used in the design of a chemical system?
- How is stoichiometry used in the analysis of a chemical system?
1.1. Big Picture
Module 7—Chemical Analysis
 Big Picture
Big Picture
Photo courtesy Kennedy Space Center/NASA
In Module 6 you explored one of the greatest achievements of humans—the ability to explore space. As you found in your study, successful space travel depends heavily on life-support systems. Many of these systems are developed using stoichiometric principles.
As you will see in this module, the careful planning and design of life-support systems must involve a knowledge of chemical systems and the quantities of substances involved.

© Alexander Raths/iStockphoto
Even if you are not planning to travel into space anytime soon, you may have concerns about the food space travellers eat or the environment in which they live. Quantitative techniques are used to monitor the nutritional content of foods and the concentration of toxic substances present in the environment.
In this module you will apply your knowledge of stoichiometry and explore how stoichiometry is used in the design and analysis of a variety of chemical systems.
1.2. In this Module
Module 7—Chemical Analysis
In This Module
Lesson 1—Limiting Reagents
The focus of this lesson is to learn how to apply your knowledge of stoichiometry to determine which reactant will be the first to deplete during a chemical reaction. Understanding the calculations used to determine the limiting reagent can be applied to a variety of situations, including specifying the quantities of reactants that should be used in a laboratory procedure that you design and determining the necessary quantities of chemicals used in a life-support system for a spacecraft.
-
How can limiting reagents be used to predict the amount of product for a chemical process?
Lesson 2—Titration Analysis
In this lesson you will learn about titration, which is a process used to determine the quantity of substance in a test sample. Although many methods can be used to determine quantity, titration is one of the most reliable and easily performed experimental procedures. You will see how your knowledge of stoichiometry can be used to analyze the data collected during a titration experiment. In addition, you will see how titration can be used to study an acid-base chemical system and other chemical systems.
-
How can titration be used to calculate the quantity of solute within a solution?
Lesson 3—Titration Curves and Selecting Indicators
In Lesson 2 you performed a titration. In this lesson you will construct a graph depicting the changes in concentration of key components in a chemical reaction. This graph is called a titration curve. You will also interpret a titration curve and identify aspects of this graph that demonstrate significant features of the chemical reaction.
In Module 5 you used indicators to determine the pH of a solution, and in Lesson 2 you used an indicator to identify the endpoint of a titration reaction. In this lesson you will learn how to use your titration curve to select appropriate indicators for a titration, allowing for precise and sensitive detection of the completion of a chemical reaction.
- How are titration graphs drawn?
- How do you select an appropriate indicator for a titration?
 Module Assessment
Module Assessment
The assessment in this module consists of three lesson assignments and a module project (Parts 1, 2, and 3).
- Module 7: Lesson 1 Assignment
- Module 7: Lesson 2 Assignment
- Module 7: Lesson 3 Assignment
- Module Project (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
-
Module Project (Part 2)—The Nature of Scientific Research and Apollo 13
- Module Project (Part 3)—Analytic Measurement Technology
1.3. Lesson 1 Intro
Module 7—Chemical Analysis
Lesson 1—Limiting Reagents
 Get Focused
Get Focused

Courtesy NASA
The Apollo 13 lunar mission was one of the defining moments in the history of space exploration. On April 11, 1970, disaster struck when one of two oxygen tanks exploded, significantly reducing the amount of breathable air available to the astronauts.
Because the spacecraft was over 300 000 km from Earth at the time of the explosion, there was no choice but for the mission to complete its scheduled course.
While the amount of oxygen remaining in the one working tank was sufficient to last the duration of the mission, there was another problem. Because of the explosion, the three astronauts had to move into the lunar module, a part of the spacecraft intended for only two people. With every breath the astronauts took, they released carbon dioxide gas into the lunar module, overwhelming the capacity of the system to remove carbon dioxide from the air. Since a higher level of carbon dioxide in the air can be toxic, the challenge facing NASA engineers on the ground was to figure out a way to control the concentration of carbon dioxide in the air inside the spacecraft using materials aboard the vessel.
How did they do it?
Essential Questions
-
How can limiting reagents be used to predict the amount of product for a chemical process?
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Save a copy of the Module 7: Lesson 1 Assignment to your course folder. You will receive more information about how to complete this assignment later in the lesson.
In this lesson you will also complete the first two parts of your module assessment:
-
Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
-
Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
These are the first two parts of your Module Assessment. Later in the lesson you will read more about the Module Assessment. Save copies of your work in your course folder. At the end of Lesson 3, you will submit all parts of the Module Assessment to your teacher.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check and Try This questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answers to all the questions in this lesson and place those answers in your course folder.
1.4. Page 2
Module 7—Chemical Analysis
 Explore
Explore
 Discuss
Discuss

Courtesy NASA
The picture on the right shows NASA engineers hard at work after the explosion. The engineers are attempting to construct a carbon dioxide filtration system for the astronauts aboard the Apollo 13 spacecraft.
What scientific skills and attitudes do you think the scientists in this photo possess? Post your thoughts on the discussion board.
Read the postings made by other students. Add any skills and attitudes you may have overlooked to your list. You will need this list later to complete Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13. You will send your list to your teacher at the end of the lesson.
 Read
Read
Limiting and Excess Reagents
In Module 6 you learned that baking powder is a combination of sodium hydrogen carbonate (baking soda) and potassium hydrogen tartrate (cream of tartar). Although these two substances are present in stoichiometric proportions in baking powder, what would be the result if one component were in excess relative to the other component?
Recall from your study in Module 5 that an excess of the sodium hydrogen carbonate—a basic compound known to have a bitter taste—could alter the taste of the food being baked. Similarly, an excess of potassium hydrogen tartrate could leave the finished product with a different undesirable taste, or it could allow for an unexpected reaction to occur with another ingredient.
Have you considered what effect a deficiency of one component relative to another might have on the quantity of carbon dioxide produced by the reaction? A cake that doesn’t rise to its desired height or texture may have been the unfortunate result of a component in the recipe being in a limited quantity relative to the other components.
How do you use your knowledge of stoichiometry to design a reaction that will ensure that it is a complete reaction?
Read “Calculating Mass of Excess Reagents” on page 320 of your textbook. Then work through “SAMPLE problem 8.1.”
 Self-Check
Self-Check
SC 1. Complete “Practice” question 2 on page 321 of your textbook.
 Self-Check Answer
Self-Check Answer
SC 1. “Practice” question 2, page 321
Zn(s)
+
2 HCl(aq)
→
ZnCl2(aq)
+
H2(g)
m = 2.00 g
MZn = 65.41g/molc = 2.00 mol/L
V = ?
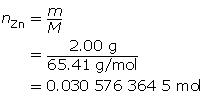
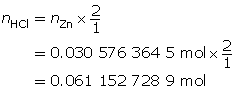
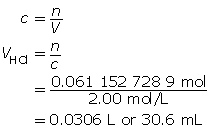
Now add 10% to this value.
30.6 mL + 3.1 mL = 33.7 mL
The volume of acid required to completely react with the zinc is 33.7 mL.
1.5. Page 3
Module 7—Chemical Analysis
 Read
Read
Have you ever ridden in a vehicle that ran out of gas before reaching the planned destination? A situation like this quickly reminds you that depleting one of the reactants in a chemical process causes the reaction to quit. Chemical reactions don’t have a fuel gauge like a vehicle does, so how can you use your knowledge of stoichiometry to identify the limiting reagent in a reaction?
Read “Identifying Limiting and Excess Reagents” on pages 321 to 323 of your textbook. Work through “SAMPLE problems” 8.2 and 8.3 and the “COMMUNICATION example.”
 Self-Check
Self-Check
SC 2. 2.50 mol of propane, C3H8(g), is combusted with 12.0 mol of oxygen gas.
-
Write the balanced chemical equation.
-
Predict the limiting and excess reagents.
-
Calculate the volume of CO2(g) produced when the reaction is complete. Assume SATP conditions.
SC 3. 10.0 g of butane, C4H10(g), is combusted with 30.0 g of oxygen gas.
-
Write the balanced chemical equation.
-
Determine the number of moles of each reactant.
-
Predict the limiting and excess reagents.
-
Calculate the mass of CO2(g) produced when the reaction is complete.
SC 4. 2.00 L of methane, CH4(g), is combusted with 9.00 L of oxygen gas. Assume STP conditions.
-
Write the balanced chemical equation.
-
Determine the number of moles of each reactant.
-
Predict the limiting and excess reagents.
-
After the reaction is complete, the atmospheric conditions change. Calculate the volume of CO2(g) produced if the atmospheric pressure is now 99.0 kPa and the temperature is 19.0°C.
SC 5. A 12.0-mL sample of a 0.500-mol/L silver nitrate solution is mixed with 10.0 mL of 0.400-mol/L NaOH(aq). Calculate the mass of precipitate produced in the reaction.
1.6. Lab
Module 7—Chemical Analysis
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
You will complete the Analysis questions for this lab as part of your Module 7: Lesson 1 Assignment. These questions appear in the first part of the Module 7: Lesson 1 Assignment that you saved to your course folder earlier in this lesson.
 Lab: Chemical Analysis Using a Graph
Lab: Chemical Analysis Using a Graph
Follow the lab instructions given in the Lesson 1 Assignment and answer the Analysis questions in your Lesson 1 Assignment.
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
Complete the remaining questions in the Module 7: Lesson 1 Assignment. After you have completed these questions, save your work to your course folder. You will submit the Lesson 1 Assignment to your teacher at the end of the lesson.
1.7. Page 5
Module 7—Chemical Analysis
 Reflect and Connect
Reflect and Connect
In this lesson you will develop Parts 1 and 2 of the Module Assessment. After you have completed both parts, save your work to your course folder. You will complete Part 3 of the Module Assessment later in this module. After you complete all three parts, you will submit the Module Assessment to your teacher.
 Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
The solution to the problem of carbon dioxide accumulating in the Apollo 13 spacecraft required the development of a new way to use equipment already aboard the spacecraft. In the living environment of the Apollo 13 spacecraft, there was not enough LiOH(s) available to fully react with all of the CO2(g) produced by the astronauts. The ingenious design that used scrubbers intended for one part of the spacecraft to be used in another part allowed the NASA engineers to ensure the safety of the astronauts.
How did NASA determine the quantity of lithium hydroxide necessary to operate the carbon dioxide scrubber system? Was a knowledge of stoichiometry required?
The carbon dioxide scrubbers used in the Apollo missions were canisters containing lithium hydroxide. Air in the spacecraft was circulated through the cylinders, allowing a reaction between lithium hydroxide and carbon dioxide to take place.
2 LiOH(s) + CO2(g) → Li2CO3(s) + H2O(l)
Use the following information to determine the mass of lithium hydroxide necessary for the duration of the mission:
-
An average adult at rest exhales 0.50 L of air with each breath and takes approximately 15 breaths per minute.
-
Approximately 4.5% of exhaled air is carbon dioxide.
-
Assume that conditions in the Apollo 13 lunar module were at SATP.
-
The Apollo 13 mission lasted for 3.7 days after the oxygen tank exploded.
-
Assume lithium hydroxide reacts completely with carbon dioxide gas.
As you develop your answer, provide details explaining how you used this information and how it impacted the answer you calculated.
 Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
Read “The Nature of Scientific Research” on pages 794 and 795 of your textbook.
-
Identify the terms and concepts listed in each of the following categories that the engineers working on the Apollo 13 mission may have utilized when developing a workable CO2 scrubber. Provide a justification for each of the terms and concepts you select.
-
Types of Studies
-
Design Factors
-
Nature of Evidence and Results
-
Reporting Research
-
STS Issues
-
Scientific Attitudes
-
-
In the discussion posting at the beginning of this lesson, you identified skills and attitudes NASA engineers might possess that help them do their job. Consider the final list of skills and attitudes you prepared with the list of terms and concepts identified in your answer to question 1 above. Identify and explain any correlations you see between these two lists.
-
Identify one other profession, apart from engineering, where a similar set of skills and attitudes is required. Provide a justification for your choice by considering the terms and concepts listed on pages 794 and 795 of the textbook.
 Assessment
Assessment
Submit the following items to your teacher:
-
Module 7: Lesson 1 Assignment
-
Discuss
Save copies of the following items in your course folder:
-
Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
-
Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
At the end of Lesson 3, you will submit all parts of the Module Assessment to your teacher.
1.8. Page 6
Module 7—Chemical Analysis
 Lesson Summary
Lesson Summary

Courtesy NASA
In this lesson you explored this essential question:
-
How can limiting reagents be used to predict the amount of product for a chemical process?
Developing a “quick fix” to control the CO2(g) concentration in the Apollo 13 spacecraft demonstrates what must be considered when chemical systems have a limited amount of one of the reactants.
As part of your Module Assessment you used stoichiometry to calculate the mass of lithium hydroxide necessary to remove excess carbon dioxide from the air inside a spacecraft on an Apollo mission. In a situation such as space travel, making careful calculations involving necessary components is essential to ensure the safety and success of the mission.
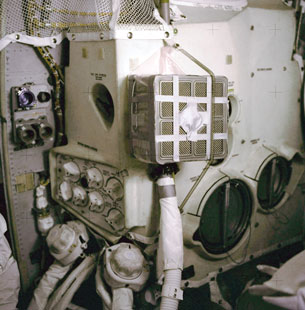
Courtesy NASA
1.9. Lesson 2 Intro
Module 7—Chemical Analysis
Lesson 2—Titration Analysis
 Get Focused
Get Focused

Photo courtesy of Kennedy Space Center/NASA
In the mid-1960s, Tang (orange drink crystals) was used by astronauts during the Gemini spaceflights. One of the reasons for using Tang on this and current space missions is that it helps mask the taste of the by-products in the water the astronauts drink. A second benefit is that Tang contains high concentrations of vitamins A and C, helping astronauts meet their daily nutritional requirements.
The nutritional information on a juice box indicates that citrus juice is a rich source of vitamin C. The average daily requirement for vitamin C is 90 mg for men and 75 mg for women. Some juice boxes indicate that the product contains 80% of the recommended daily intake for vitamin C. If the claim on the juice box is true, how much vitamin C must be present in the juice solution?
How can you verify that the quantity of vitamin C you calculated is actually present in the juice? The obvious answer is that you would have to test the juice. As a consumer, you may have wondered if you are really getting what you are paying for.
How can you test juice to determine the concentration of vitamin C in it? In this lesson you will learn about titration—a process routinely used to determine the concentration of substances in a solution.
Essential Questions
-
How can titration be used to calculate the quantity of solute in a solution?
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
Save a copy of the Module 7: Lesson 2 Assignment to your course folder. You will receive more information about how to complete this assignment later in the lesson.
You will also work on Module Assessment (Part 3)—Analytic Measurement Technology. This is the third part of your Module Assessment. Save a copy of your work in your course folder. At the end of Lesson 3, you will submit all parts of this the Module Assessment to your teacher.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.10. Page 2
Module 7—Chemical Analysis
 Explore
Explore
 Read
Read
Titration Analysis
titration: a method of analyzing a solution by adding known amounts of a standardized solution until an endpoint is produced
A titration is a method of analyzing a solution by adding known amounts of a standardized solution until an endpoint is produced. This laboratory procedure is used to determine the concentration of a solute in a solution being tested. In Lesson 1 you studied the limiting reagent and how it influences the predictions made using stoichiometry. A titration involves the careful addition of one reactant to another to determine the exact point where one reactant becomes excess.
Read page 328 of “8.4 Titration Analysis” in your textbook to become familiar with some of the terms used in a titration analysis.
 Watch and Listen
Watch and Listen
View the video “Performing a Titration.” Pay careful attention to how the equipment is used during this procedure.
Review the bolded terms shown on page 328 of your textbook. Match each of the following terms from the video with one of the terms from your textbook.
Term from the Video |
|
Term from the Textbook |
1. green solution colour |
|
A. titrant |
2. standard solution of 0.100-mol/L NaOH(aq) |
|
B. sample |
3. 10.00-mL acid solution |
|
C. endpoint |
 Watch and Listen Answers
Watch and Listen Answers
- C
- A
- B
Reading the level of the solution in the burette is an essential skill. To practise this skill, view the video a second time, pausing the video so you are able to read the solution level in the burette at the start and at the end of the trial. Compare the burette readings you made to the values stated in the video.

In the “Performing a Titration” video, you watched NaOH(aq) solution being added to an HCl(aq) acid solution until a permanent change in the colour of the bromothymol blue indicator occurred. How does the colour change for bromothymol blue signal the detection of the end of the reaction?
Recall from your study in Module 4 that bromothymol blue appears yellow in acidic solutions and blue in basic solutions. As shown in the video, a titration proceeds until an endpoint is reached. In this case, the excess NaOH(aq) added after all the acid in the sample had reacted creates a basic solution, causing the indicator to change colour.
 Try This
Try This
The following data was collected during the titration shown in the “Performing a Titration” video. Use this data to determine the concentration of HCl(aq) in the sample tested.
Reactant |
HCl(aq) |
NaOH(aq) |
Location |
Erlenmeyer flask |
burette |
Volume |
10.00 mL |
13.89 mL |
Concentration of Reactant |
? |
0.100 mol/L |
 Try This Answer
Try This Answer
HCl(aq) |
+ |
NaOH(aq) |
→ |
H2O(l) |
+ |
NaCl(aq) |
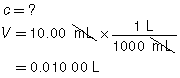 |
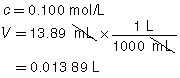 |
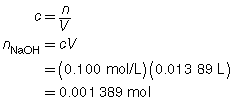
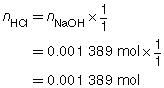
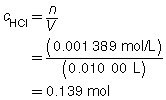
The concentration of the HCl(aq) solution is 0.139 mol/L.
1.11. Page 3
Module 7—Chemical Analysis
 Read
Read

© Laurence Gough/shutterstock
Titrations can provide very accurate data. One means to ensure accuracy is by doing multiple trials. Multiple trials allow for selecting the most consistent trials for further analysis. One sign of consistency is obtaining a similar endpoint colour between trials.
Work through “SAMPLE problem 8.4” on page 329 of your textbook to see how titration data from multiple trials is analyzed.
After working through this sample problem, you may wonder why only three trials were required in this titration. As stated earlier, a titration is repeated until consistent results are obtained, such as endpoint colour and titrant volumes that are within 0.2 mL of each other.
 Self-Check
Self-Check
SC 1. A 50.0-mL sample of HCl(aq) is titrated with 0.300-mol/L NaOH(aq). When the endpoint is reached, 35.0 mL of the titrant has been used. Calculate the concentration of the hydrochloric acid based on this one trial.
SC 2. A titration is performed to determine the concentration of KOH(aq) in a 40.0-mL sample. The endpoint is reached when 18.02 mL of 0.100-mol/L HNO3(aq) is added. Calculate the concentration of KOH(aq) based on this trial. If the manufacturer’s label for KOH(aq) says the concentration is 0.0480 mol/L, what is the percent difference for the experimental result?
SC 3. A 20.0-mL sample of calcium hydroxide is titrated with 0.100-mol/L hydrochloric acid until the endpoint is reached. The volume of titrant used for each trial is shown in the following table.
Trial |
1 |
2 |
3 |
Final Burette Reading (mL) |
22.7 |
45.2 |
67.6 |
Initial Burette Reading (mL) |
0.4 |
22.7 |
45.2 |
Using this data, calculate the concentration of the Ca(OH)2(aq).
 Self-Check Answers
Self-Check Answers
SC 1.
HCl(aq) |
+ |
NaOH(aq) |
→ |
H2O(l) |
+ |
NaCl(aq) |
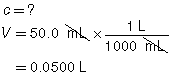 |
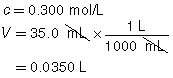 |
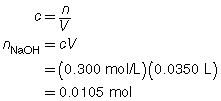
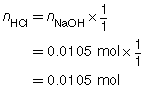
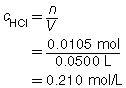
The concentration of the hydrochloric acid is 0.210 mol/L.
SC 2.
HNO3(aq) |
+ |
KOH(aq) |
→ |
KNO3(aq) |
+ |
H2O(l) |
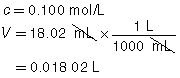 |
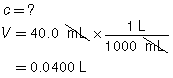 |
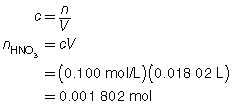
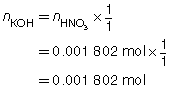
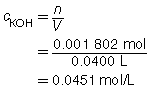
Based on the one trial, the concentration of KOH(aq) is 0.0451 mol/L.

SC 3.
Trial |
1 |
2 |
3 |
Final Burette Reading (mL) |
22.7 |
45.2 |
67.6 |
Initial Burette Reading (mL) |
0.4 |
22.7 |
45.2 |
Volume of Titrant (mL) |
22.3 |
22.5 |
22.4 |
The three volumes agree within 0.2 mL and are used to calculate the average volume of HCl(aq) required.
![]()
Ca(OH)2(aq) |
+ |
2 HCl(aq) |
→ |
CaCl2(aq) |
+ |
2 H2O(l) |
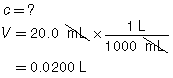 |
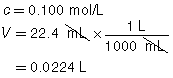 |
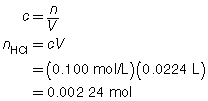
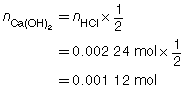
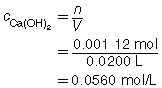
The concentration of Ca(OH)2(aq) is 0.0560 mol/L.
1.12. Lab
Module 7—Chemical Analysis
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
The data from the following lab will be used to complete your Module 7: Lesson 2 Assignment.
 Lab: Titration of an Acid with a Base
Lab: Titration of an Acid with a Base
So far in this lesson you have observed how titrations are performed. In this activity, you will complete a virtual lab investigation where you will perform a titration and analyze the data you collect. You will use the data from this investigation to complete your Module 7: Lesson 2 Assignment.
After you complete the titration lab, use the data to calculate the concentration of the hydroiodic acid solution tested.
Retrieve the Module 7: Lesson 2 Assignment that you saved to your course folder earlier in the lesson, and record your answers under question 1.
![]() Post a copy of your analysis to the discussion board, and review the postings of two other students. Provide comments to your classmates about the organization, clarity, and precision of their answers. Record your comments, along with your calculations, under question 2 in your Module 7: Lesson 2 Assignment. Save a copy of your Lesson 2 Assignment to your course folder.
Post a copy of your analysis to the discussion board, and review the postings of two other students. Provide comments to your classmates about the organization, clarity, and precision of their answers. Record your comments, along with your calculations, under question 2 in your Module 7: Lesson 2 Assignment. Save a copy of your Lesson 2 Assignment to your course folder.
1.13. Lab
Module 7—Chemical Analysis
 Reflect and Connect
Reflect and Connect
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
The data from the following lab will be used to complete your Module 7: Lesson 2 Assignment.
 Lab: Determining the Quantity of Vitamin C in Fruit Juice
Lab: Determining the Quantity of Vitamin C in Fruit Juice
A titration does not always have to involve an acid-base reaction. Analyze the information provided about a titration to determine the concentration of vitamin C in fruit juice.
Save the document Determining the Quantity of Vitamin C in Fruit Juice to your course folder. Use the data provided to answer the Analysis questions in your Module 7: Lesson 2 Assignment.
Save a copy of your Module 7: Lesson 2 Assignment to your course folder.
 Reflect on the Big Picture
Reflect on the Big Picture
 Module Assessment (Part 3)—Analytic Measurement Technology
Module Assessment (Part 3)—Analytic Measurement Technology
In this lesson you learned that titration analysis is a method of determining the amount of concentration of a substance. Other techniques are also available to detect small amounts of solute. What use would such technology have in spaceflight and exploration?
Read “Case Study: Analytic Measurement Technology” on pages 337 and 338 of your textbook. Answer the “Case Study Questions” on page 338. When answering these questions, consider more than one perspective.
Read “2.1 Science and Technology in Society” on pages 44 and 45 to review information about different perspectives prior to completing your response.
Submit a copy of your answer to your teacher and place a second copy of your answer in your course folder.
 Assessment
Assessment
Submit the following item to your teacher:
- Module 7: Lesson 1 Assignment
Save a copy of this item in your course folder:
- Module Assessment (Part 3)—Analytic Measurement Technology
At the end of Lesson 3, you will submit all parts of the Module Assessment to your teacher.
1.14. Page 6
Module 7—Chemical Analysis
 Lesson Summary
Lesson Summary
In this lesson you explored the following essential question:
- How can titration be used to calculate the quantity of solute in a solution?
You performed a titration and analyzed the data collected during a titration experiment. Titration analysis can be used to verify the concentration of a substance in a solution or commercial product. Through your work in determining the mass of vitamin C in a juice box, you learned that the techniques involved in titrations can be used in situations other than acid-base reactions.
In the next lesson you will learn more about the design of a titration experiment and why it allows for precise determination of a solute’s concentration.
Lesson Glossary
titration: a method of analyzing a solution by adding known amounts of a standardized solution until an endpoint is produced
1.15. Lesson 3 Intro
Module 7—Chemical Analysis
Lesson 3—Titration Curves and Selecting Indicators
 Get Focused
Get Focused

Courtesy of Johnson Space Center/NASA
Legend has it that Neil Armstrong, the first man to walk on the Moon, was reluctant to go into space unless Aspirin was included on the mission. Aspirin tablets—used for relieving headaches and muscle pain—were included in the medical kit (shown here) of the Apollo 11 space capsule and for later space missions. The key ingredient in Aspirin is acetylsalicylic acid (ASA).
As you have discovered, titration is a process that can be used to determine the quantity of a substance in a sample. In this lesson you will learn more about the stoichiometry involved in the process of titration and why titration can provide such accurate and reliable data in quantitative analyses. You will also perform a titration of pain-relief tablets to determine the mass of ASA present.
Essential Questions
-
How are titration graphs drawn?
-
How do you select an appropriate indicator for a titration?
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Save a copy of the Module 7: Lesson 3 Assignment to your course folder. Later in the lesson you will receive more information about how to complete this assignment and when to submit it to your teacher.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.16. Page 2
Module 7—Chemical Analysis
 Explore
Explore
 Read
Read
In Lesson 2 you performed a titration of an acid using a base. During that process, many changes occurred within the chemical system, some of which you were able to see and others you were not. In this lesson you will apply your knowledge of stoichiometry to explain the changes that occur within a chemical system during a titration. To begin, read the first paragraph of “8.5 Acid-Base Titration Curves and Indicators” on page 333 of your textbook.
Titration Curves
titration curve: a graph of the change in pH of the system being studied as standardized solution is added
A titration curve is a graph of the change in pH of the system being studied as a standardized solution is added. Recall that a titration involves adding small amounts of titrant to the test solution. What if you had the chance to assess the changes in the system after each addition of titrant—what would you want to measure? What would the pattern of the change look like?
 Try This
Try This
Drawing a Titration Curve
In this activity you will draw a titration curve using the data you generate in a virtual titration experiment. Combining acids and bases causes neutralization and, therefore, a change in the pH of the solution. To monitor the progress of your titration, you will record pH throughout the titration.
Purpose
You will draw a titration curve.
Problem
What is the shape of a titration curve?
Procedure
Step 1: Construct a data table like the following.
Volume of Titrant Added |
pH of Test Solution |
Indicator Colour |
|
|
|
Title the data table “Titration of HCl(aq) with 1.0-mol/L NaOH(aq).”
Step 2: Open the “Titration of an Acid” virtual lab.
Step 3: Read the instructions and complete Procedures 1 and 2 as described in the instructions. As you proceed through the titration, record your observations in the appropriate places in your data table.
Step 4: Use graph paper or a spreadsheet program to construct a graph of pH (vertical axis) versus Volume of 1.0-mol/L NaOH(aq) Added (horizontal axis).
Step 5: On the graph, label where the colour change of the phenolphthalein indicator occurred.
Step 6: Add a suitable title, and ensure the features of your graph are appropriately labelled. Save your work in your course folder.
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Copy your titration curve from the Try This activity above into the space provided for question 1 in the Module 7: Lesson 3 Assignment that you saved to your course folder at the beginning of the lesson. Save your Module 7: Lesson 3 Assignment to your course folder.
1.17. Page 3
Module 7—Chemical Analysis
 Read
Read
The unique shape of a titration curve demonstrates that important changes occur. Interpreting the different parts of a titration curve is an important skill. Read the first four paragraphs of “Interpreting Titration pH Curves” on pages 334 and 335 of the textbook.
 Self-Check
Self-Check
SC 1. Retrieve the extra copy of the titration curve you saved to your course folder. Find the equivalence point and equivalence point pH for each titration curve. Using your labelled graphs, state the equivalence point pH and volume of titrant required to reach the equivalence point for each titration. Save your answer for use later in this lesson.
![]() Self-Check Answer
Self-Check Answer
SC 1.
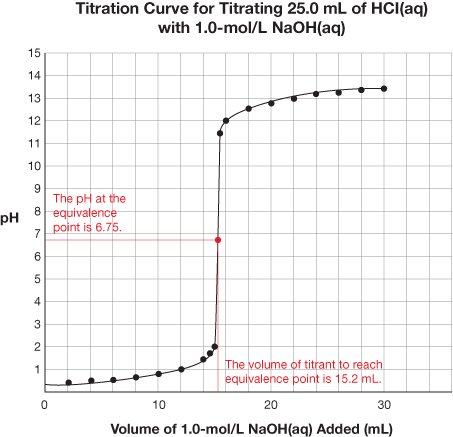
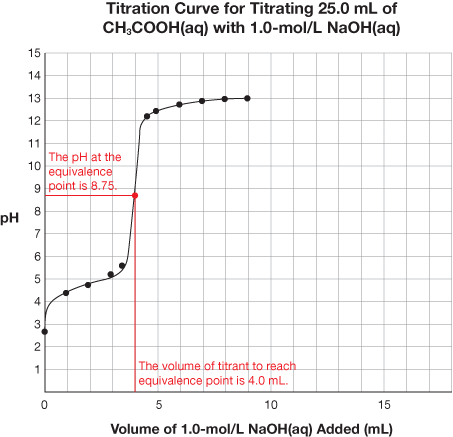
Titration |
Equivalence Point pH |
Volume of Titrant to Reach Equivalence Point |
HCl(aq) vs. NaOH(aq) |
6.75 |
15.2 mL |
CH3COOH(aq) vs. NaOH(aq) |
8.75 |
4.0 mL |
 Read
Read
Read the rest of “Interpreting Titration pH Curves” on page 335 of your textbook.
You will note that a titration curve conveys a great deal of information about the chemical reaction that occurred to produce this data.
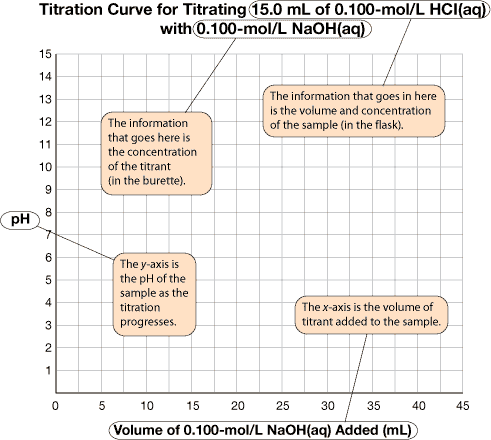
Axes Labels: The x-axis represents the volume of titrant added to the sample (usually in mL). The y-axis represents the pH of the sample as the titration progresses.
Title: The title identifies the reactants.
A very important piece of information on the graph is the equivalence point. The equivalence point represents the end of the reaction and is located at the midpoint of the steep middle region. While the titration graph for a strong monoprotic acid reacting with a strong monoprotic base will have an equivalence point pH of 7, this is not necessarily the case for weak acids or weak bases being titrated.
The volume of titrant required to reach the equivalence point is the value used to complete calculations like those you have completed in the previous lessons.
The titration data and the completed titration curve are shown here for comparison.
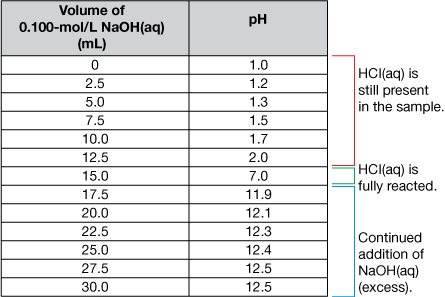
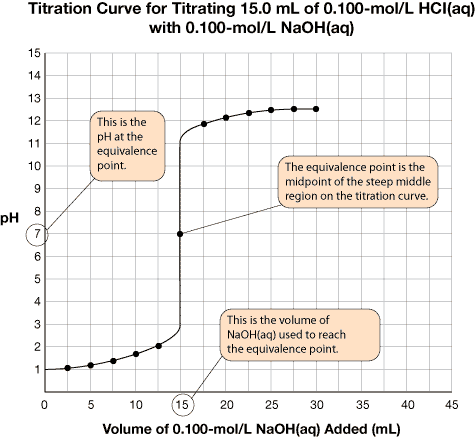
Selecting Indicators for a Titration Curve
If you titrate HCl(aq) with NaOH(aq)—two colourless solutions—without using an indicator, there will be no colour change to signify that the equivalence point has been reached. Indicators respond to changes in pH and provide a dramatic colour change to signify the endpoint of a titration. But with so many indicators to choose from, which one is best?
Read “Choosing Acid-Base Indicators for Titration” on page 336 of your textbook.
A good rule of thumb for selecting an indicator is that the midpoint pH of the range of colour change is equal to the equivalence point pH.
 Self-Check
Self-Check
SC 2. Retrieve the copy of the titration curve that you used in SC 1. Use the information provided in the “Acid-Base Indicators” table in the Chemistry Data Booklet to identify suitable indicators that will signify the endpoint for the titration shown. Then indicate the colour change that would signify the endpoint of the titration.
 Self-Check Answer
Self-Check Answer
SC 2.
Titration |
Suitable Indicator |
pH of Indicator |
Colour Change at Endpoint |
HCl(aq) vs. NaOH(aq) |
bromothymol blue |
6–7.6
6–8.0 |
green (intermediate between yellow to blue) orange (intermediate between yellow and red) |
CH3COOH(aq) vs. NaOH(aq) |
thymol blue phenolphthalein |
8–9.6
8–10.0 |
green (intermediate between yellow to blue) pink |
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Retrieve the Module 7: Lesson 3 Assignment you saved earlier in this lesson. Complete questions 2, 3, and 4. Save a copy of your assignment to your course folder.
1.18. Page 4
Module 7—Chemical Analysis
 Reflect and Connect
Reflect and Connect
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Titration Analysis of ASA
Retrieve your saved copy of the Module 7: Lesson 3 Assignment. Follow the instructions in Titration Analysis of ASA, and then complete the Analysis questions. Save a copy of your assignment to your course folder. Your completed Lesson 3 Assignment will be submitted to your teacher at the end of the lesson.
 Going Beyond
Going Beyond
© Mike Rogal/shutterstock
Acetylsalicylic acid (ASA) has many other effects on the human body in addition to the ability to relieve pain. As a result, ASA is often prescribed by doctors to treat patients with a variety of ailments. What are some of the other uses for ASA? Are there risks in prescribing the use of ASA for so many people?
Search the Internet to identify risks associated with ASA. Which risks are of concern to children, young adults, and older adults? How might these risks influence your choice of medication?
Save a copy of the list of risks associated with ASA to your course folder. Include comments on how these risks might influence your use of ASA in the future.
 Assessment
Assessment
Submit the following items to your teacher:
- Module 7: Lesson 3 Assignment
-
Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
-
Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
-
Module Assessment (Part 3)—Analytic Measurement Technology
1.19. Page 5
Module 7—Chemical Analysis
 Lesson Summary
Lesson Summary
In this lesson you extended your knowledge of titrations by learning how to analyze titration curves. The data for a titration curve can be obtained by using a pH meter to record the pH while a sample is being titrated. One of the most important parts of a titration curve is the equivalence point, located at the midpoint of the steep middle region. The equivalence point signifies the completion of the reaction. The volume of titrant required to reach the equivalence point is used to complete calculations involved in quantitative analysis. The pH at the equivalence point is used to select an appropriate indicator for the titration. In Reflect and Connect, you applied your knowledge of titration analysis to determine the amount of ASA (acetylsalicylic acid) present in a tablet of Aspirin.
Lesson Glossary
titration curve: a graph of the change in pH of the system being studied as standardized solution is added
1.20. Module Summary/Assessment
Module 7—Chemical Analysis
Module Summary and Assessment
 Module Summary
Module Summary
Quantitative analysis involves a variety of techniques that rely on stoichiometric relationships between substances involved in a reaction. In this module you performed a titration using an acid and a base. You also performed a titration to determine the quantity of vitamin C in fruit juice. By studying some of the considerations necessary to support a journey into space, you considered the following questions:
-
How is stoichiometry used in the design of a chemical system?
-
How is stoichiometry used in the analysis of a chemical system?
These questions are relevant to more than spaceflight, since the principles of stoichiometry can be applied to any process in which different components combine and form into new arrangements. Think about this the next time you are preparing sandwiches or planning a picnic for a large group of people. You may be surprised to find yourself thinking in ratios that relate the number of people attending to the number of plates required or the number of veggie burgers to be prepared.
 Module Assessment
Module Assessment
The assessment in this module consists of the following:
-
Assignments for Lessons 1, 2, and 3
-
Module Assessment (Part 1)—Using Stoichiometry to Save the Apollo 13 Astronauts
-
Module Assessment (Part 2)—The Nature of Scientific Research and Apollo 13
-
Module Assessment (Part 3)—Analytic Measurement Technology
1.21. Module Glossary
Module 7—Chemical Analysis
Module Glossary
titration: a method of analyzing a solution by adding known amounts of a standardized solution until an endpoint is produced
titration curve: a graph of the change in pH of the system being studied as standardized solution is added