Module 2
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 2 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 10:52 AM |
Description
Created by IMSreader
1. Module 2
Module 2—Talking Energy
Introduction

© designalldone/shutterstock
In Module 2 you will keep investigating energy changes in chemical reactions by focusing on changes that occur at the molecular level.
You will continue the ecotour planning you began in Module 1 by considering using biodiesel in vehicles required by your ecotour. Biodiesel is an alternative fuel that can be produced from waste cooking or other oils. You will consider the production and use of biodiesel from a variety of perspectives.
In Module 2 you will investigate the following question:
- What energy changes must be considered when designing chemical systems?
You will also continue to address the Module 1 questions:
- Should energy be given a higher priority when making decisions about society’s future?
- How does society use the energy of chemical changes?
- What are the impacts of energy use on the environment?
- How does society use knowledge of the energy associated with chemical processes to promote sustainability?
- In what ways have issues of energy use affected the development of past and present societies?
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 2, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit A Concept Organizer. In the Module 1 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 2—Talking Energy
 Big Picture
Big Picture

You may have planned that participants in your ecotour will see parts of your local area by cycling. What mode of transportation could be more ecofriendly than cycling—transportation powered by people? However, the cyclists in your ecotour will likely need to be supported by a vehicle. If so, you might consider using a vehicle that uses an environmentally friendly fuel as its energy source.
Biodiesel is one such fuel that has been receiving a great deal of attention. Oil from the canola plants pictured to the right of the cyclists in the photograph can be used to produce biodiesel, as can waste vegetable oil from restaurants.
In Module 2 you will learn more about energy changes in chemical reactions while investigating the production, testing, and use of biodiesel as a possible fuel source for your ecotour.
The use of biodiesel as a vehicle fuel for your ecotour planning will tie into the Module 2 Assessment. Read on to learn more about the lessons, activities, and assessments you will complete as you progress through Module 2.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 2 Assessment you will research biodiesel, and you will use your findings to decide whether to use biodiesel in your ecotour operation. You will develop a presentation for your ecotour clients and others interested in your operation explaining why you will or will not use biodiesel.
You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 2—Talking Energy
In This Module
Lesson 1—Alternative Fuels: Biodiesel
When running an ecotour you often have to “walk the talk.” Using biodiesel where possible may improve your credibility as an ecotour operator. In Lesson 1 you will make and test biodiesel, and you will begin to consider whether you should use biodiesel in your ecotour’s operations.
You will investigate the following lesson question:
- What makes a good fuel?
Lesson 2—Energy Diagrams and Activation Energy
In Lesson 2 you will consider the events that occur during a chemical reaction from the molecular level. You will learn about activation energy, and you will integrate this concept into your understanding of enthalpy changes for reactions involving endothermic and exothermic change.
You will investigate the following lesson question:
- Why is energy needed to start an exothermic reaction?
Lesson 3—Catalysts
Catalysts are important components of industrial and biological systems. In Lesson 3 you will learn how catalysts affect the rate of chemical reactions, including the production of biodiesel.
You will investigate the following lesson questions:
- What is a catalyst?
- How does a catalyst influence a chemical reaction?
1.3. Lesson 1
Module 2—Talking Energy
Lesson 1—Alternative Fuels: Biodiesel

© Sophia Winters/shutterstock
 Get Focused
Get Focused
There is no doubt that your ecotour will involve some sort of travel using vehicles. Whether you will be driving support for the participants when they are cycling or collecting them downstream after river canoeing, you will need to transport people and equipment to and from the unique activities you will offer.
Many ecotours have made the decision to use biodiesel in their vehicles where possible. What is biodiesel and how does its use promote sustainability? In Lesson 1 you will learn about biodiesel. You will begin to evaluate its properties and suitability as a fuel for your ecotour. To assist in your evaluation you will make and test biodiesel.
Consider the following question as you complete Lesson 1:
- What makes a good fuel?

 Module 2: Lesson 1 Assignment
Module 2: Lesson 1 Assignment
In the Lesson 1 Assignment you will complete a virtual investigation in which you will make and test biodiesel. Download a copy of the Module 2: Lesson 1 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 2—Talking Energy
 Explore
Explore
 Try This
Try This
What makes a good fuel?
TR 1. List as many criteria as you can think of that should be considered in choosing an appropriate fuel for a vehicle. Rank your criteria in order of importance, and then rationalize your ranking.
Save your response in your course folder. You may wish to revise your ranking as you work through Module 2 and learn more about different fuels, including biodiesel.
 Read
Read
How many of the criteria you listed in TR 1 relate to the energy content of fuel? In Module 1 you performed a calorimetry experiment to determine the energy content per gram of a nut. You also performed calculations to determine the molar heat of combustion for some hydrocarbons using standard molar enthalpies of formation.
Calorimetry and standard molar enthalpy of formation are two ways to determine the energy change associated with the combustion of a fuel. How are these two methods similar and how are they different? Which means would be most helpful to you when making a decision about using biodiesel as a vehicle fuel? To answer these questions it might be helpful to know what biodiesel is. In the next investigation you will make biodiesel and then test it to determine its energy content.
In preparation for the investigation, complete the following Self-Check questions to review your ability to perform calculations for molar enthalpy of combustion. You may wish to review Module 1, Lesson 5, SC 1 prior to attempting these questions.
 Self-Check
Self-Check
Use the following data to answer the next question:
Fuel |
Heat of Combustion (kJ/g) |
Chemical Formula |
methane |
50.01 |
CH4 |
propane |
46.36 |
C3H8 |
octane |
44.43 |
C8H18 |
SC 1. Rank the fuels from lowest to highest heat of combustion as expressed in kJ/g in the data table.
SC 2. Explain how you could convert a heat of combustion for the hydrocarbons listed in the data table into a molar enthalpy of combustion.
SC 3. Calculate the molar enthalpy of combustion for each of the fuels listed in the data table. Rank the fuels from lowest to highest molar enthalpy of combustion.
SC 4. Compare the rankings you developed in SC 1 and SC 3.
SC 5. Does a trend exist between the molar enthalpies of combustion and the molar masses of the fuels? (You may wish to refer to the work you completed in SC 3 and SC 4.)
SC 6. List one advantage of communicating the energy change associated with combustion of a hydrocarbon using the units kJ/mol and kJ/g.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. ranking: octane, propane, methane
- SC 2. To convert from kJ/g to kJ/mol, multiply the heat of combustion by the molar mass of the hydrocarbon. For example
- –50.00 kJ/g × 16.05 g/mol = –802.5 kJ/mol
–46.34 kJ/g × 44.11 g = –2043.9 kJ/mol
–44.408 kJ/g × 114.26 g = –5074.1 kJ/mol
SC 3.
methane: CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
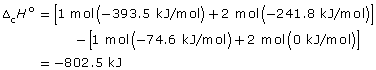
![]()
propane: C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g)
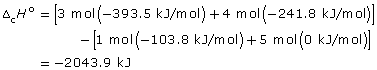
![]()
octane: C8H18(g) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(g)
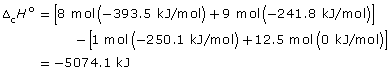
![]()
ranking: methane, propane, octane
SC 4. For the hydrocarbons being compared, the rankings are reversed between the lists. The hydrocarbon with the highest heat of combustion has the lowest molar heat of combustion.
SC 5.
| Hydrocarbon | Molar Enthalpy of Combustion (kJ/mol) |
Molar Mass (g/mol) |
methane, CH4 |
–802.5 |
16.05 |
propane, C3H8 |
–2043.9 |
44.11 |
octane, C8H18 |
–5074.1 |
114.26 |
Trend: As the molar mass of a hydrocarbon increases, so does its molar enthalpy of combustion.
SC 6. Fuels are usually not sold or distributed by the mole. Volume or mass of a fuel is measurable. An energy value per unit mass can be easily used in predicting net energy output from a selected mass.
Molar energy changes are convenient because they provide insight into the energy associated with a defined number of fuel particles (one mole). When expressed per unit of mass, the number of reacting particles can be different.
1.5. Page 3
Module 2—Talking Energy
 Lesson 1 Lab: Biodiesel
Lesson 1 Lab: Biodiesel
Introduction

One of the ways to evaluate biodiesel as a fuel is to prepare some biodiesel and then test it. Biodiesel can be prepared from any vegetable oil or animal fat. A popular choice of oil is waste vegetable oil used in food preparation. In this investigation you will make biodiesel from olive oil.
This lab is in two parts. You will prepare and test biodiesel against the materials used in its preparation to compare their qualitative aspects of combustion. You will also perform an experiment to determine the biodiesel's energy content.
![]() Retrieve your copy of the Module 2: Lesson 1 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 1 Assignment.
Retrieve your copy of the Module 2: Lesson 1 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 1 Assignment.
![]() If you do not have access to all materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the following two virtual investigations:
If you do not have access to all materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the following two virtual investigations:
If you will not be completing the lab yourself, remember to record data and observations as you view the presentations.
Background Information
Previous science courses probably taught you that molecules store chemical potential energy, which can be released when chemical bonds in matter are broken and new bonds are formed. Maybe you’ve even completed an experiment to determine the energy in a sample of food or fuel. Whether oil is crude or plant-based—peanut oil, palm oil, olive oil, or canola oil—it has molecules that are often described as “energy rich.”
Rudolf Diesel, the inventor of the diesel engine, demonstrated in 1900 at the World’s Fair in Paris that an engine could run on peanut oil rather than on petroleum-based fuels.
Considering the current concern over the depletion of crude oil reserves and the growing demand for energy, Diesel was quite a visionary when he mentioned, in 1912, that
Even though the use of vegetable oils for engine fuels may seem insignificant today, such oils may become in the course of time as important as the petroleum and coal tar products of the present time.
Diesel’s insight about the potential of plant-based oils as an energy source is being demonstrated by people using waste vegetable oil from restaurants as a fuel in their vehicles powered by diesel engines. Around the world, some governments and some corporations within the energy industry are promoting the production of biodiesel, the fuel produced by the modification of vegetable oils.
The yearly consumption of diesel fuel in North America is 245.5 billion litres, most of it for transportation of goods and people. The production and sale of blended diesel fuels, which contain a percentage of biodiesel (e.g., 20% in the case of biodiesel B-20), mixed with petroleum-based diesel fuel, is a growing industry in Europe, where sources of petroleum-diesel are limited. Concerns about depleting petroleum sources elsewhere in the world, including North America, have forced governments and the oil industry to consider biodiesel as a means to offset some of the demand for petroleum-diesel.
In this project, you will investigate the production and use of biodiesel, focusing on the chemistry of its production, the practicality of its use at low temperatures, the energy changes associated with its use, and the sustainability of this energy source.
Performing the Lab
Complete the following two investigations:
- Instructions for Lesson 1 Lab: Part 1—Making Biodiesel
- Instructions for Lesson 1 Lab: Part 2—Testing Biodiesel
 Module 2: Lesson 1 Assignment
Module 2: Lesson 1 Assignment
If you have not already done so, retrieve your copy of the Module 2: Lesson 1 Assignment that you saved to your computer earlier in this lesson. Complete the assignment and save it in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
1.6. Page 4
Module 2—Talking Energy
 Read
Read
In the analysis for the investigation you were asked to calculate an energy of combustion per gram of biodiesel combusted. The biodiesel that you made in the investigation is not a pure substance. The biodiesel contained many products including different organic acids, glycerol, and products of incomplete or side reactions. Can you explain why the mixture of combustible materials in biodiesel makes it impossible to calculate the biodiesel’s molar mass and a molar enthalpy for its combustion? Can you think of other examples where it is not possible to calculate an energy content per mole of substance?
 Reflect on the Big Picture
Reflect on the Big Picture
Throughout Module 2, Reflect on the Big Picture activities will prepare you for the Module 2 Assessment. In the Module 2 Assessment you will prepare a presentation defending a position on the use of biodiesel by your ecotour company.
In this lesson you completed a virtual investigation in which you prepared biodiesel and tested it to determine its energy of combustion per gram. At the beginning of this lesson you were asked to consider the possible use of biodiesel as a fuel for vehicles used in your ecotour. Does biodiesel measure up?
RBP 1. Begin the process of assessing biodiesel as a fuel. Use the knowledge you gained from this lesson and research you find on the Internet and through other sources to consider the following qualities of biodiesel:
- energy content relative to other fuels
- availability relative to other fuels
- cleanliness of combustion
- ease of preparation
You may wish to consider these qualities from different perspectives as you develop your response. For example, the availability of biodiesel may be seen differently from an ecological or economic perspective as opposed to a scientific one.
You may also use this opportunity to address other factors important to your decision. For example, there may be considerations that are important because of where your tour will operate, the conditions under which it will operate, or specific activities that you have planned.
Save your work in your ecotour planning subfolder. You will consider other aspects of biodiesel use and production, and you will refine and add to your work as you progress through Module 2.
 Module 2: Lesson 1 Assignment
Module 2: Lesson 1 Assignment
Submit your completed Module 2: Lesson 1 Assignment to your teacher.
1.7. Page 5
Module 2—Talking Energy
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- What makes a good fuel?
This lesson addressed the criteria and perspectives society uses to make fuel choices. In running an ecotour you often have to “walk the talk.” Using biodiesel where possible may improve your credibility as an ecotour operator and may enable you to provide a more sustainable experience for your participants. You prepared and tested biodiesel, and you reflected on the criteria used to decide whether to use biodiesel. This will help you make an appropriate choice of fuel at the end of Module 2.
1.8. Lesson 2
Module 2—Thinking Energy
Lesson 2—Energy Diagrams and Activation Energy
 Get Focused
Get Focused

© Johanna Goodyear/shutterstock
In Lesson 6 of Module 1 you learned how potential energy diagrams can be used to communicate enthalpy changes. It is common knowledge that you have to provide a spark, a flame, or energy in another form to get most fuels to combust.
In Lesson 1 of Module 2 you observed that some of the fuels being tested in the spirit lighters were much easier to ignite than others. Using potential energy diagrams, how can you demonstrate the energy necessary to initiate a combustion and the difference in the magnitude of energy required for some fuels?
In Lesson 2 you will investigate how energy is involved in initiating chemical change. In order to better evaluate biodiesel as a fuel, it is necessary to understand the factors that affect how easily some fuels ignite. Your investigation in this lesson may also provide insight into the scientific reasoning behind the suggested practice of storing fuels in a cool, dark place.
Consider the following question as you complete Lesson 2:
- Why is energy needed to start an exothermic reaction?
 Module 2: Lesson 2 Assignment
Module 2: Lesson 2 Assignment
There is no assignment for this lesson. However, later in this lesson you will continue your work on the Module 2 Assessment as you collect information about the performance of biodiesel compared to traditional diesel fuel.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.9. Page 2
Module 2—Thinking Energy
 Explore
Explore
 Read
Read
In previous science courses you learned that chemical reactions occur as a result of colliding particles. To understand why a chemical reaction occurs and what events occur during a reaction, you may have to imagine a reaction in very slow motion.
Read pages 524–526 in the textbook to learn about the events that occur during a chemical reaction.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–3 on page 526 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
The molecules must have sufficient energy and be in the proper orientation when they collide. If these two conditions are met, a reaction can occur.
Practice 2.
Scientific: A better understanding of how chemical reactions occur might improve the ability to predict when chemical reactions might occur, including the effect that catalysts and other substances have on the progress of a reaction.
Technological: A better understanding of how chemical reactions occur may lead to the development of new processes that improve efficiency or reduce the generation of by-products.
Practice 3.
- N2(g) + 2 O2(g) → 2 NO2(g) ΔfHº = +66.4 kJ
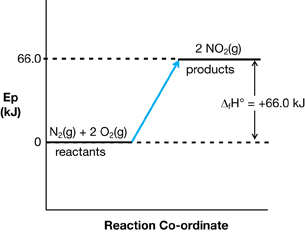
- Answers may vary. The following hypotheses are possible answers:
-
The electrons or electrical field in the area of a lightning strike provide conditions that are favourable to initiating the reaction.
-
Electrons from the lightning may act as a catalyst.
-
Electrons or the electrical field from the lightning may ionize surrounding particles, allowing them to become catalysts in the chemical reaction between nitrogen and oxygen.
-
 Read
Read
So what does a spark really do in terms of starting a combustion reaction? In the previous section you learned that molecules must collide with sufficient energy to cause a reaction. From where do the molecules get that energy?
As you may have suspected, energy to initiate a combustion reaction can come from a spark, the flame from a match, or, in the case of a spontaneous combustion, from the heat of a hot day. Read the section “Activation Energy of a Reaction” on pages 526–530 in the textbook to learn more about the energy required to initiate a reaction.
 Try This
Try This
Answer the following question in which you will interpret and apply your textbook reading.
TR 1. It is a common observation that vapourized hydrocarbons combust more readily than liquid hydrocarbons. Use a potential energy diagram to compare the potential energy of a vapourized hydrocarbon to the potential energy of the same hydrocarbon in the liquid phase. In addition, use the difference in potential energy between the two phases to explain this observation and how it relates to energy of activation within a chemical system.
Save your response in your course folder and submit a copy to your teacher.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 2–6 on page 531.
 Self-Check
Self-Check
SC 3. Complete “Section 12.2” questions 1–3 on page 534. After completing the questions, ask yourself the following question: Is activation energy only required to initiate an exothermic reaction like a combustion of a hydrocarbon?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Section 12.2 1.
Bond energy is the energy required to break a chemical bond, and it is the energy released when a chemical bond is formed.
Section 12.2 2.
In the reactant molecules, if the bonds that are being broken are strong, the activation energy is likely to be high. If the bonds being broken are weak, it is likely that the activation energy will be relatively low.
Section 12.2 3.
By definition, a chemical reaction involves a rearrangement of bonds to produce new substances in which atoms are conserved but the bonding is different. This can only happen if at least some bonds are broken and new ones are formed. For example, in the decomposition of water, O-H bonds are broken and H-H and O=O bonds are formed.
1.10. Page 3
Module 2—Thinking Energy
 Reflect on the Big Picture
Reflect on the Big Picture
In Module 2, Reflect on the Big Picture will prepare you for the Module 2 Assessment in which you will prepare a presentation defending a position on the use of biodiesel by your ecotour company.
In the previous lesson you saw that biodiesel requires more effort to light compared to methanol. Since biodiesel is a mixture of molecules, it is difficult to make conclusions about its activation energy relative to other hydrocarbons.
In your investigation about the performance of biodiesel you may have already found that biodiesel performs very similarly to diesel fuel in terms of engine operation and fuel economy. Is this true at all temperatures? If your ecotour involves winter activities, will using biodiesel present a problem?
Research how biodiesel performs compared to conventional diesel fuel and how biodiesel performs at temperatures similar to those you will encounter when operating your ecotour. Remember to consider the qualities you researched in Lesson 1 as you compare the two fuels.
Save your notes in your ecotour planning sub-folder. Remember to collect information that is accurate (correct) and reliable (consistent with the results from other or repeated tests). You will be required to use the information you collect to support your position on the use of biodiesel, so it is important to consider the source and the number of sources from which you collect your information. Remember that you will refine and add to your research as you progress through Module 2.
 Module 2: Lesson 2 Assignment
Module 2: Lesson 2 Assignment
There is no assignment for this lesson.
1.11. Page 4
Module 2—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following question:
- Why is energy needed to start an exothermic reaction?
You learned about activation energy and the events that occur during a chemical reaction. You learned that certain conditions, including sufficient particle speed and orientation, are necessary for a reaction to proceed. Energy of activation is a threshold that, once met, allows molecules to collide with sufficient energy to react.
In this lesson you also learned how the concept of activation energy integrates into your understanding of enthalpy changes for both endothermic and exothermic changes in chemical reactions. Finally you learned how to view enthalpy changes as a change in the bond energies between reactant and product particles.
Potential Energy Diagram for an Exothermic Reaction
Potential Energy Diagram for an Endothermic Change
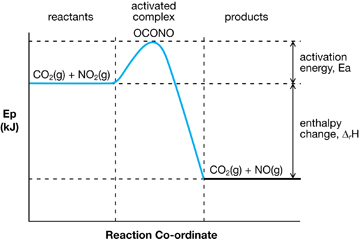
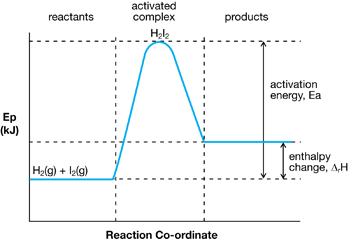
In the next lesson you will further your understanding of activation energy and its impact on chemical processes as you learn about catalysts. You will apply your understanding of catalysts as you further assess the production of biodiesel through a chemical reaction in which sodium hydroxide is used as a catalyst.
1.12. Lesson 3
Module 2—Talking Energy
Lesson 3—Catalysts
 Get Focused
Get Focused

As you researched the performance of biodiesel in the previous lesson, did you come across personal accounts from people who have been making and using biodiesel in their own automobiles? You probably came across information that identifies the importance of using a strong base—a hydroxide—when preparing biodiesel. Why is sodium hydroxide, or alternatively potassium hydroxide, essential to making biodiesel? As you will learn in this lesson, these compounds act as catalysts in the chemical reaction that produces biodiesel. Catalysts are the focus of this lesson.
Consider the following questions as you complete Lesson 3:
- What is a catalyst?
- How does a catalyst influence a chemical reaction?
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
In the Lesson 3 assignment you will design a laboratory investigation to compare catalysts used in the preparation of biodiesel. Download a copy of the Module 2: Lesson 3 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. You will also continue your work on the Module 2 Assessment by completing a Reflect on the Big Picture exercise.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.13. Page 2
Module 2—Talking Energy
 Explore
Explore
 Read
Read
In the previous lesson you learned about activation energy, the energy required to initiate a chemical reaction. You considered the combustion of hydrocarbons and the energy required to “activate” molecules. You learned that once the reacting particles have reached the necessary activation energy, the chemical reaction, in this case a combustion reaction, can occur.
In Module 2 Lessons 1 and 2, you learned that molecules need to have sufficient energy and must collide in the correct orientation in order for the bond between atoms in the reactants to be broken and for new bonds to form. For some chemical reactions, the energy required to initiate chemical change can be quite high. As you learned in Lesson 1, reactions with higher activation energies tend to occur more slowly. If you are depending on a reaction, and need it to occur quickly, how can you overcome the problem of a high activation energy slowing your reaction rate?
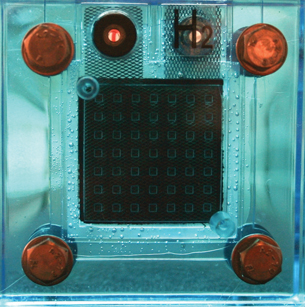
© chiara levi/iStockphoto
You may have learned about fuel cells in previous science courses. Many fuel cells appear to use a reaction that looks like a combustion reaction. For example, the hydrogen-oxygen fuel cell used in the space program relies on the reaction 2 H2(g) + O2(g) → 2 H2O(l).
This reaction is also the basis for a hydrogen “pop” test you may have performed in previous science courses. When performing a hydrogen pop test, a burning splint is placed into a test tube containing a small quantity of hydrogen gas. As you learned earlier, the flame serves as a source of energy. The energy helps the reactants achieve the activation energy required for the reactants to engage in collision and form the products of the reaction.
In the space program hydrogen and oxygen are the reactants in a fuel cell—a device that converts chemical potential energy into electrical energy. The electricity produced by the fuel cells in a spacecraft run all the major systems inside the spacecraft including lighting, communication, and life support. Due to the risk of explosion, the reaction between hydrogen and oxygen cannot involve a spark flame, but it still requires an input of energy to proceed. How can this be done without a flame?
catalyst: a substance that reduces the energy necessary to start a chemical reaction
Catalysts are substances that increase the rate of a chemical process but are not consumed during the process.
Look at the fuel cell pictured. You will see a membrane in the middle of the cell. The chemical reaction between hydrogen and oxygen in the fuel cell occurs on this membrane. Using a structure like this allows the reaction between hydrogen and oxygen to occur without the need for any more energy than the gases already have at room temperature. Since the membrane in a fuel cell allows the reaction between hydrogen and oxygen to occur with a lower activation energy, the membrane acts as a catalyst.
A catalyst is something within a chemical system that lowers the activation energy required for a reaction to occur by allowing an alternate path for the reaction. This last point is very well demonstrated by the fuel cell membrane, since the membrane acts to physically separate the hydrogen and oxygen gases, meaning they never actually collide with one another. By using a catalyst, a different mechanism for the reaction occurs, and the product H2O(l) is made.
Many industrial processes, including the production of biodiesel, involve the use of catalysts. In industrial settings catalysts are often used because they increase the rate (speed) of a reaction without being consumed in the overall process. Often, they also allow a reaction to occur at a lower temperature than would otherwise be possible.
Read pages 535–538 in the textbook. Stop reading when you get to the section “Catalysts and the Nature of Science.” As you read this section, pay special attention to the explanation of how a catalyst affects the changes to the potential energy in a chemical system as a reaction occurs.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–4 on page 535 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
Development of catalysts has primarily occurred using empirical knowledge involving investigations that appear to try conditions and identify changes needed to improve the reaction rate.
Practice 2.
The catalytic converter and catalytic cracking towers in petroleum refining are examples of technological inventions that involved the use of catalysts.
Practice 3.
Photosynthesis, fermentation, and cellular respiration are natural (biological) processes that involve the use of catalysts. Many other examples exist as most biological reactions involve the use of enzymes as a catalyst.
Practice 4.
Since enzymes are a type of catalyst they are not changed by the chemical reaction. Since enzymes catalyze many reactions, they do not have to be present in large numbers to improve the efficiency of a chemical process in the body. If poisoned, the reaction rate will be dramatically affected since very few enzyme molecules exist. The resulting rate of reaction will be dramatically reduced due to fewer catalyzed reactions.
 Read
Read
You might wonder how catalysts are able to reduce the activation energy, which increases the rate of a chemical reaction, and still not be consumed in the process. Read the section “Theoretical Explanation of Catalysts” on pages 536–538 in the textbook.
The effect catalysts have on chemical systems is of great importance to many industries. Read the sections “Catalysts and the Nature of Science” and “Uses of Catalysts” on page 538–541 in the textbook.
 Try This
Try This
TR 1. Read the four points made in the “Summary” section on page 541 of the textbook. For each of the four points listed, provide some elaboration explaining why each of these statements made about catalysts is true. You may choose to provide an explanation or an example from the textbook or these course materials in a written, audio, or some other format.
 Self-Check
Self-Check
SC 2. Complete “Section 12.3” questions 1–6 and 9 on page 542 of the textbook.
SC 3. In addition to completing the “Section 12.3” questions listed above, explain how your answers to these questions will help you determine the desirability of burning biodiesel as a fuel.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Section 12.3 1.
Same: the reactants, products, potential energy (position of reactants and products on a potential energy diagram), overall enthalpy change
Different: activation energy, rate (speed) of reaction, reaction intermediates, activated complex that occurs during the reaction
Section 12.3 2.
a. C and D
b. A, B, and E
c. F
d. 2 A + B + E → F
Section 12.3 3.
a. The activation energy for the forward reaction is 60 kJ.
b. The activation energy for the reverse reaction is 95 kJ.
c. The enthalpy change for the forward reaction is −35 kJ.
d. The enthalpy change for the reverse reaction is +35 kJ.
e. The forward reaction is exothermic.
Section 12.3 4.
If only 55 kJ of kinetic energy were available, the reactant molecules would rebound without reacting successfully.
Section 12.3 5.
In the reverse direction, a collision with kinetic energy equivalent to 55 kJ would also result in rebound and an unsuccessful reaction.
Section 12.3 6.
The following are examples of catalysts providing solutions to technological problems:
- The catalytic converter in a vehicle’s exhaust system reduces air pollution by catalyzing the reduction of NO(g) and NO2(g) to harmless N2(g).
- Catalysts are used in the refining of petroleum to increase production of the more desirable fractions by catalytic reforming or alkylation.
- In the upgrading of bitumen to synthetic crude, catalysts are employed during the hydrocracking and hydrotreating processes.
- In the brewing industry, the enzyme in yeast is used in the conversion by fermentation of sugar to ethanol.
Section 12.3 9.
Risks
- Burning fossil fuels is the biggest contributor to global warming through the dramatic increase in CO2 levels in the atmosphere.
- Fossil fuels are non-renewable, so the world will eventually face shortages.
- The crude oil could be put to better use making petrochemicals instead of being burnt.
Benefits
- Crude oil continues to be less expensive than other sources of energy.
- Canada has a secure supply of crude oil and bitumen for the foreseeable future.
- Crude oil is the most practical and convenient source of liquid fuels for vehicles.
1.14. Page 3
Module 2—Talking Energy
 Reflect and Connect
Reflect and Connect
There is no doubt that you would use a catalyst if you were to make biodiesel. The procedure you followed in Lesson 1 indicated that either sodium hydroxide or potassium hydroxide could be used. Does it matter which one of these you use?
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
Retrieve your copy of the Module 2: Lesson 3 Assignment you saved to your computer earlier in this lesson. Complete the Assignment.
Save a copy of your completed Assignment in your course folder. You will receive instructions later in this lesson on when to submit your work to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture
In Module 2, Reflect on the Big Picture will prepare you for the Module 2 Assessment in which you will prepare a presentation defending a position on the use of biodiesel by your ecotour company.
So far in this module you have collected information about the following qualities:
- energy content of biodiesel relative to other fuels
- availability of biodiesel relative to other fuels
- cleanliness of biodiesel when combused (emissions testing)
- ease of preparation of biodiesel
- performance of biodiesel under weather conditions in the area in which your ecotour will occur
The final quality you will consider is the catalyst used in the preparation of biodiesel.
RBP 1. Consider the chemical properties of sodium hydroxide or potassium hydroxide, and collect information about the cost and environmental issues related to using these as catalysts to prepare biodiesel. Identify safety issues that you would need to attend to when preparing or using biodiesel.
Save your research in your ecotour planning sub-folder. After you complete this lesson, proceed to the Module 2 Summary and Assessment where you will use the information you have collected to complete the Module 2 Assessment.
 Module 2: Lesson 3 Assignment
Module 2: Lesson 3 Assignment
Submit your Module 2: Lesson 3 Assignment to your teacher.
1.15. Page 4
Module 2—Talking Energy
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- What is a catalyst?
- How does a catalyst influence a chemical reaction?
Catalysts are important components of industrial and biological systems. The use of catalysts increases the rate at which a reaction occurs, thus allowing for the more rapid production of products. In this lesson you saw how catalysts allow unique chemical systems like fuel cells and petroleum cracking to occur with significantly reduced energy input. You also evaluated the use of a catalyst in the production of biodiesel from more perspectives than just efficiency and rate of reaction. You considered cost of production, environmental aspects, and safety concerns.
1.16. Module Summary/Assessment
Module 2—Talking Energy
 Module Summary
Module Summary
This module completes your study of thermochemical changes that began in Module 1.
You considered the following module questions, some of which you began to examine in Module 1:
- What energy changes must be considered when designing chemical systems?
- Should energy be given a higher priority when making decisions about society’s future?
- How does society use the energy of chemical changes?
- What are the impacts of energy use on the environment?
- How does society use knowledge of the energy associated with chemical processes to promote sustainability?
- In what ways have issues of energy use affected the development of past and present societies?
In Module 2 you investigated energy changes in chemical reactions by focusing on changes that occur at the molecular level. You learned about activation energy and its role in every chemical reaction, and you learned about the conditions necessary for molecules to participate in a chemical reaction. You also learned about catalysts and their ability to lower activation energy and thereby increase the rate of a chemical reaction.
You completed a virtual investigation in which you made and tested biodiesel, and you researched biodiesel’s use as a vehicle fuel for your ecotour. The information you learned in Module 2 will provide a foundation for your learning in other units of this course, especially in Unit D where you will investigate equilibrium of chemical systems.
Concept Map or Graphic Organizer
As you worked through Module 2, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 2 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 2 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
Evaluating the Use of Biodiesel in Your Ecotour
In this module you were asked to consider the use of biodiesel as a vehicle fuel in your ecotour. Does biodiesel measure up? Undertake an assessment based on the following qualities:
- energy content relative to other fuels
- availability relative to other fuels
- cleanliness of combustion
- ease of preparation
- performance under weather conditions such as those in which your ecotour will operate
- cost, safety, and environmental issues related to the use of a catalyst in the preparation of biodiesel
You may wish to consider these qualities from different perspectives as you develop your response. For example, the availability of biodiesel may be seen differently from an ecological or economic perspective than from a scientific viewpoint.
You may also wish to address other factors important to your decision. For example, there may be considerations that are important because of where your tour will operate, the conditions under which it will operate, or specific activities that you have planned.
Your response should be a persuasive argument. Think of questions that partners or investors in your ecotour might ask. Work answers to these questions into your presentation. To identify your tour’s fuel needs and some of the questions you should address, review all of the information in your ecotour planning sub-folder, including the information you collected in Module 1. Remember to include the sources of your information in your presentation.
Select a means to convey your message that you feel will be most suitable to your goals and audience. This may be as text (in the form of a brochure) or in a multimedia format such as an audio, video, or slide presentation. This is your chance to capture the attention of people interested in your ecotour and to demonstrate how you are able to apply your knowledge of chemical energetics and ecology to your ecotour planning.
![]() Post a copy of your presentation to the discussion area for your class. Review the presentations of at least two other students. Provide feedback to the students to help them improve their presentations. You may choose to revise your presentation based on the feedback you receive from your classmates. Save a copy of your revised response in your course folder.
Post a copy of your presentation to the discussion area for your class. Review the presentations of at least two other students. Provide feedback to the students to help them improve their presentations. You may choose to revise your presentation based on the feedback you receive from your classmates. Save a copy of your revised response in your course folder.
Consider the criteria and descriptions in the scoring guide below as you develop and revise your presentation. Your teacher will use these same criteria to assess the work you submit. Save the final version of your presentation in your course folder and submit a copy to your teacher for assessment.
Your Module 2 Assessment will also form part of your Unit A Assessment. Please see the Unit A Assessment for more information.
Score |
Scoring Description |
| 4 Standard of Excellence |
The response is well organized and addresses all the major points of the question using effective, appropriate, and clear communication strategies. The interrelationships between science, technology, and society are thoroughly understood. Risks and benefits of biodiesel preparation and use are thoroughly evaluated.
Descriptions, explanations, and/or interrelationships between the concepts provided are correct and reflect a thorough understanding of the use of biodiesel. Insightful and convincing arguments are used to support a decision or judgment, and a range of perspectives is considered. |
3 |
The response is organized and addresses most major points of the question using effective, appropriate, and clear communication strategies. The interrelationships between science, technology, and society are understood. Risks and benefits of biodiesel preparation and use are evaluated.
Descriptions, explanations, and/or interrelationships between the concepts provided are correct and reflect an understanding of the use of biodiesel. Clear and logical arguments are used to support a decision or judgment, and alternative perspectives are considered. |
2 |
The response is generally organized and addresses most of the major points of the question using adequate communication strategies. The interrelationships between science, technology, and society are generally understood. Risks and benefits of biodiesel preparation and use are listed.
Descriptions between the concepts provided are generally correct and reflect an adequate understanding of the use of biodiesel. Logical arguments are used to support a decision or judgment, and perspectives are considered. |
| 1 | The response is poorly organized and addresses few major points of the question. The interrelationships between science, technology, and society are poorly understood. Few risks and benefits of biodiesel preparation and use are listed.
Descriptions, explanations, and/or interrelationships between the concepts provided are incorrect and reflect a poor understanding of the use of biodiesel. Poorly formed arguments are used to support a decision or judgment, and only one point of view is considered. |
| 0 | The response does not address any of the major points of the question at an appropriate level for a 30-level course. |
1.17. Module Glossary
Module 2—Talking Energy
Module Glossary
catalyst: a substance that reduces the energy necessary to start a chemical reaction
Catalysts are substances that increase the rate of a chemical process but are not consumed during the process.