Module 3
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 3 |
| Printed by: | Guest user |
| Date: | Thursday, 30 October 2025, 10:25 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 3
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Page 6
- 1.9. Lesson 2
- 1.10. Page 2
- 1.11. Page 3
- 1.12. Page 4
- 1.13. Page 5
- 1.14. Lesson 3
- 1.15. Page 2
- 1.16. Page 3
- 1.17. Page 4
- 1.18. Page 5
- 1.19. Lesson 4
- 1.20. Page 2
- 1.21. Page 3
- 1.22. Page 4
- 1.23. Page 5
- 1.24. Page 6
- 1.25. Page 7
- 1.26. Lesson 5
- 1.27. Page 2
- 1.28. Page 3
- 1.29. Page 4
- 1.30. Page 5
- 1.31. Module Summary/Assessment
- 1.32. Module Glossary
1. Module 3
Module 3—Electrochemical Reactions
Module Introduction

top left: © Mikhail Olykainen/shutterstock
top middle: © Paul Picone/shutterstock
all others: © 2008 Jupiterimages Corporation
Metals are fantastic materials to use for structural components or for decoration. Sometimes, however, metals react when they are exposed to air, water, or other substances. In some situations the reaction decreases the performance of the object. In extreme cases, metal fatigue or failure can have drastic consequences, such as when metal fatigue causes a large structure to collapse.
A great deal of scientific work and technological development has gone into studying metals and into preventing metal corrosion. If you consider how many of the objects you use involve metals, you will understand why so much attention is placed on understanding corrosion.
In Module 3 you will investigate the following questions:
- What properties of metals make them popular choices in the construction and production of materials?
- How can an understanding of corrosion allow for better selection of materials and for development of methods that reduce material damage?
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 3, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit B Concept Organizer. In the Module 3 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 3—Electrochemical Reactions
 Big Picture
Big Picture

In 1995, the High Level Bridge in Edmonton, Alberta, underwent a major restoration in which approximately 45 tonnes of rusted steel were replaced, and 100 000 litres of paint were applied. How could so much rust form? Why was paint applied at the end of the restoration?
Look around and you will see many metal objects. Metals are heavily used by society because of their properties. For example, metals are electrical conductors, are strong, and can be made into different shapes.
One negative property of metals is their reactivity. The performance of metals can be negatively affected when metals undergo chemical change. Metal performance is an issue in large structures such as bridges and in small devices that use metals to conduct electric current.
You will learn many theoretical concepts in Module 3. It is important that you identify where you are able to use these theoretical concepts to predict and explain your observations and where you are not able to do so.
In Module 4 you will investigate many technological applications of the main theoretical concept of Module 3—reduction-oxidation reactions. Successfully applying your theoretical knowledge to new situations will help you in Module 4.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 3 Assessment you will apply your knowledge of electrochemical change to describe the electrochemical basis behind methods of preventing corrosion.
You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 3—Electrochemical Reactions
In This Module
Lesson 1—Electrochemical Change
In Lesson 1 you will observe some chemical reactions in a new way—as electrochemical changes. You will learn to write chemical reactions that demonstrate the kind of change that occurs in these systems, and you will learn how to interpret these reactions.
You will investigate the following lesson question:
- What is an electrochemical change?
Lesson 2—Predicting Redox Reactions
In Lesson 2 you will analyze sets of data collected from the reaction of metals with aqueous metal ions to identify patterns in the reactivity of metals and their ions. You will use these patterns to predict reactions involving other substances.
You will investigate the following lesson questions:
- Why do some metals appear to react more easily than other metals?
- Is it possible to predict whether an electrochemical reaction will occur spontaneously?
Lesson 3—Half-Reactions
In Lesson 3 you will learn to write and use half-reactions involving more than one oxidizing or reducing agent. You will use half-reactions and net ionic equations to explain chemical change in systems in which the half-reactions for the oxidizing and reducing agents are not provided to you.
You will investigate the following lesson questions:
- How can combinations of species act together as oxidizing or reducing agents?
- Can half-reactions be used to predict and explain changes that occur within a chemical system?
- Can the same substance be the oxidizing agent and the reducing agent in an electrochemical process?
Lesson 4—Oxidation Numbers and Corrosion Protection
In Lesson 4 you will interpret empirical changes in terms of oxidation states (sometimes as distinctive as colour changes by certain metal ions) or numbers used to explain the oxidation or reduction of atoms.
You will investigate the following lesson questions:
- What are oxidation numbers, and how can they be used to understand redox reactions?
- What factors cause corrosion?
- How can corrosion be prevented?
Lesson 5—Redox Stoichiometry
In Lesson 5 you will learn how to adapt your knowledge of redox reactions into designing and performing quantitative analysis. You will investigate quantitative relationships using a titration experiment involving oxidizing and reducing agents. In Module 4 you will extend this to understanding other quantitative measures.
You will investigate the following lesson questions:
- How can a chemical system be analyzed using redox reactions?
- How is the stoichiometric method applied to redox systems?
1.3. Lesson 1
Module 3—Electrochemical Reactions
Lesson 1—Electrochemical Change

© 2008 Jupiterimages Corporation
 Get Focused
Get Focused
Corrosion is a common sight on metal objects. Often we think of corrosion as the rusting of iron, but it can also be used to describe the change in other metals, like the copper in the statue shown in the picture.
What colour does the metal copper have? Can you explain what has happened to the copper in the statue? How could the copper metal change into copper ions, which appear to wash away from the statue? Is this an example of a chemical reaction and, if so, what happens when atoms change in this manner?
The type of change observed here, in which atoms appear to change form and charge, is an important type of change called electrochemical change. Electrochemical change is the focus of this unit.
Consider the following question as you complete Lesson 1:
- What is an electrochemical change?
 Module 1: Lesson 1 Assignment
Module 1: Lesson 1 Assignment
There is no assignment for this lesson.
There are other questions in this lesson that are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 3—Electrochemical Reactions
 Explore
Explore
 Try This
Try This
Have you ever noticed when you look at the jar of spare change in your house that pennies really loose their shine? Is there a relationship between the age of the pennies and their loss of luster (shininess)? Look at the dates on some of the pennies in your household change jar or in your pocket to see if there is a connection between a penny's age and its luster.
TR 1. Write a hypothesis describing a relationship between a possible factor and the loss of luster by copper as demonstrated by the pennies in your household change jar. Submit a copy of your hypothesis to your teacher for feedback.
 Read
Read
Read page 556 of the textbook.
Do you think the change in copper observed in the pennies or in the photograph of the copper statue at the beginning of this lesson demonstrates an electrochemical process? Start your analysis of these systems by writing the chemical formula for the forms of copper that have the appearances you observed. Can you describe the differences between the chemical formulae for these forms of copper? Do the differences in the chemical formulae you have written fit the description of an electrochemical process (a type of chemical change that involves a transfer of electrons)?
 Read
Read
In previous science courses you learned that the net charge given to an atom or group of atoms is due to the quantity of protons and electrons the atom or group possesses. Electrochemical changes involve a transfer of electrons. Therefore, a change in the net charge of a substance often indicates that the substance has undergone an electrochemical change.
In the case of copper, its various forms have distinct colours, and the change in copper from its elemental form (net charge zero) to copper(II) ion (net charge 2+) involves a loss of electrons to create the positively charged copper(II) ion.
Some of the first investigations involving electrochemical reactions occurred as society began to work with metals. Read pages 558–559 of the textbook to see how metallurgy developed some of the terminology you will use throughout this unit.
1.5. Page 3
Module 3—Electrochemical Reactions
 Lesson 1 Lab: Observing the Reactivity of Zinc
Lesson 1 Lab: Observing the Reactivity of Zinc
What happens when a metal is involved in a chemical reaction?
![]() View the virtual investigation “Observing the Reactivity of Zinc.” You will observe the changes that occur when zinc reacts with a solution of silver nitrate. Record your observations and answer the Analysis questions as you work through this investigation. Save a copy of your work in your course folder.
View the virtual investigation “Observing the Reactivity of Zinc.” You will observe the changes that occur when zinc reacts with a solution of silver nitrate. Record your observations and answer the Analysis questions as you work through this investigation. Save a copy of your work in your course folder.
Analysis
- Write the balanced chemical equation for the reaction of zinc and aqueous silver nitrate.
- Indicate what empirical evidence you observed in the investigation that supports the equation you have written.
- Identify which substances have undergone change in this reaction? Is the change the substance(s) underwent an electrochemical change involving a transfer of electrons? Support your reasoning.
In the next activity, you will read and work through “Sample problem 13.1” in the textbook. You will be prompted to check your answers to the Analysis Questions after completing your reading.
1.6. Page 4
Module 3—Electrochemical Reactions
 Read
Read
Electrochemical change involves a transfer of electrons. In the virtual investigation, “Observing the Reactivity of Zinc,” you saw that silver ions must have gained electrons to become solid silver. How is such a change represented?
Read pages 561–564 of the textbook to learn how to represent electrochemical changes using a balanced chemical equation. Carefully work through “Sample problem 13.1” and “Communication example 1” on page 563 to practise using chemical equations to explain electrochemical changes.
Remember to use this reading (particularly “Sample problem 13.1”) to check your answers to the analysis questions for the virtual investigation, “Observing the Reactivity of Zinc.” If you identify significant differences between your answers and the textbook reading, you may wish to contact your teacher for help.
Half-Reactions
In the textbook reading you will have noticed that half-reactions are used to describe the changes observed in chemical systems. Half-reactions are one type of balanced chemical equation. You will recall from previous science courses that balanced chemical equations demonstrate two conservation laws:
- law of conservation of mass—in any physical or chemical change, the total initial mass of reactant(s) is equal to the total final mass of product(s)
- law of conservation of charges—in any isolated system, the sum of all of the charges remains constant
Conservation of mass is quite easy to identify in an equation because the type and numbers of each type of atom are the same on both sides of the equation.
- For example,
- Ag+(aq) + 1 e– → Ag(s)
Reactants |
Products |
Ag = 1 |
Ag = 1 |
Conservation of charge involves adding up the charges on each side of the equation. If the net charge on each side is equal, the equation is balanced for charge.
- For example,
- Cu(s) → Cu2+(aq) + 2 e–
Reactants |
Products |
Cu = 1 |
Cu = 1 |
charge = 0 |
charge = +2 + (–2) = 0 |
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 7–11 on page 564 of the textbook.
SC 2. Use the table “Colours of Common Aqueous Ions” on page 11 of the Chemistry 30 Data Booklet or a similar table to determine the colour of the resulting solution if nitric acid were reacted with each of the following metals:
- nickel
- copper
- chromium
SC 3. Write balanced half-reactions for the oxidation and reduction that occurred in the virtual investigation “Observing the Reactivity of Zinc.”
1.7. Page 5
Module 3—Electrochemical Reactions
 Reflect on the Big Picture
Reflect on the Big Picture
Understanding electrochemical reactions is an important part of successfully using metals as structural materials. In this lesson you have seen how metals can undergo electron transfer reactions.
RBP 1. How do metals tend to behave in electrochemical reactions? What possible consequence might a redox reaction involving a metal have to the substance's integrity? Can you think of ways that electrochemical reactions of metals can be prevented?
Save your response in your course folder.
 Module 3: Lesson 1 Assignment
Module 3: Lesson 1 Assignment
There is no assignment in this lesson.
1.8. Page 6
Module 3—Electrochemical Reactions
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following question:
- What is an electrochemical change?
By observing the reaction between zinc and aqueous silver ions you were able to see evidence of a chemical reaction that can be explained by an electron transfer between reactants. You also learned how to write, balance, and interpret half-reactions that describe reduction or oxidation processes.
In the next lesson you will investigate electrochemical changes involving other metals, and you will conduct an experiment to investigate the difference in the reactivity of selected metals.
1.9. Lesson 2
Module 3—Electrochemical Reactions
Lesson 2—Predicting Redox Reactions
 Get Focused
Get Focused

Rust on a household strainer. Was the selection of iron a good choice of metal for the strainer considering the intended use for this object?
Each metal differs in its ability to react. For example, when exposed to air, pennies (primarily made of copper) tend to lose their luster. Silver coins and jewelry tend to tarnish and turn black. Gold, however, does not appear to lose its luster when exposed to air.
In Lesson 1 you learned that the loss of luster in some metals is due to an electrochemical reaction in which the metals are oxidized and exchange electrons with another reactant. Knowledge of which metals have the greatest potential to oxidize, and in what conditions their oxidation is rooted, could be a great asset when considering materials for use in construction or other applications.
Consider the following questions as you complete Lesson 2:
- Why do some metals appear to react more easily than other metals?
- Is it possible to predict whether an electrochemical reaction will occur spontaneously?
The focus of Lesson 2 is on learning how to interpret reaction data that you will collect. You will also learn to construct tables that will help you make predictions about the reactivity of metals or metal ions with other substances.
Being able to interpret reaction data in the manner you will learn in this lesson is essential to your study in the rest of this unit—be extra careful as you complete questions and work through problems in this lesson, and ensure you have mastered this skill before moving on to the next lesson.
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
Download a copy of the Module 3: Lesson 2 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. The assignment has the following parts:
- Part 1: Lab—Spontaneity of Redox Reactions
- Part 2: Lab Exercise 13.A on page 572 of the textbook
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.10. Page 2
Module 3—Electrochemical Reactions
 Read
Read
The relative reactivity or stability of a metal is determined empirically. Later in this lesson you will complete an investigation in which you will observe and interpret the data from many systems of metals and aqueous solutions of metal ions.
To prepare for the investigation, read page 568 and the top half of page 569 in the textbook. You will learn about the terms oxidizing agent and reducing agent. These terms are commonly used to discuss reactants involved in an electrochemical reaction.
Evidence of a chemical reaction can take many forms. List observations that indicate that a chemical reaction has taken place.
spontaneous: a process that occurs without any external energy source
A spontaneous process will occur on its own.
non-spontaneous: a process that is incapable of proceeding unless driven by an outside source of energy
When a colour change, evolution of a gas, formation of a precipitate, or temperature change of a system is observed, the reaction can be described as spontaneous; that is, the chemical change occurs without a continuous input of energy. If no reaction evidence is observed, the reaction is non-spontaneous.
In the next investigation you will determine whether the reaction between various metals and solutions of metal ions is spontaneous or non-spontaneous.
1.11. Page 3
Module 3—Electrochemical Reactions
 Lesson 2 Lab: Spontaneity of Redox Reactions
Lesson 2 Lab: Spontaneity of Redox Reactions
Read “Investigation 13.2” on page 602 in the textbook. In the virtual investigation you will complete, lead and the solution of lead(II) ion were removed. Exposure to lead and lead compounds is a concern. You may wish to consult an MSDS (Materials Safety Data Sheet) or other reference on safety in science laboratories for more information about the safety concerns associated with lead or other substances you may encounter in this course.
![]() Retrieve your copy of the Module 3: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in Part 1 of the Assignment document.
Retrieve your copy of the Module 3: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in Part 1 of the Assignment document.
![]() View the virtual investigation “Spontaneity of Redox Reactions.”
View the virtual investigation “Spontaneity of Redox Reactions.”
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
If you have not already done so, retrieve your copy of the Module 3: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete Part 1. You will receive information later in the lesson on when to complete the rest of the Lesson 2 Assignment and on when to submit your work to your teacher.
1.12. Page 4
Module 3—Electrochemical Reactions
 Read
Read
Metals that are more likely to react with the ions of other metals are considered to be more reactive or, using the terminology introduced in this lesson, are stronger reducing agents. A redox table is used to list oxidizing and reducing agents by their strengths. Read the lower half of page 569 to the end of the “Summary” section on page 571 of the textbook to learn more about the organization of a redox table.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–3 and 5–10 on page 571 of the textbook.
 Read
Read
In SC 1 you were asked to develop a hypothesis about the design of a redox table and to test the redox table using the data available to you.
Read pages 572–574 of the textbook. Carefully work through “Sample problem 13.4” on page 573 and “Figure 6” in the margin of page 573 to understand the spontaneity rule, a general principle demonstrated by redox tables constructed in the manner described in this lesson. You may wish to refer to page 7 of the Chemistry Data Booklet on which an extended redox table is shown.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 11–14 on page 573 of the textbook.
SC 3. Complete “Practice” question 20 on page 574 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 11.
Co2+(aq) + 2 e- ↔ Co(s)
Zn2+(aq) + 2 e- ↔ Zn(s)
Mg2+(aq) + 2 e- ↔ Mg(s)
Practice 12.
Cu2+(aq) + 2 e- ↔ Cu(s)
2 H+(aq) + 2 e- ↔ H2(g)
Cd2+(aq) + 2 e- ↔ Cd(s)
Be2+(aq) + 2 e- ↔ Be(s)
Ca2+(aq) + 2 e- ↔ Ca(s)
Practice 13.
The spontaneity rule is based on empirical evidence from experimental results.
Practice 14.
Cl2(g) + 2 e- ↔ 2 Cl-(aq)
Br2(g) + 2 e- ↔ 2 Br-(aq)
Ag+(aq) + e- ↔ Ag(s)
I2(s) + 2 e- ↔ 2 I-(aq)
Cu2+(aq) + 2 e- ↔ Cu(s)
SC 3.
Practice 20.
a. spontaneous
b. non-spontaneous
c. non-spontaneous
d. spontaneous
e. spontaneous
f. spontaneous
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
Retrieve your copy of the Module 3: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 of the Assignment. Save your work in your course folder. You will receive instructions later in this lesson on when to submit your completed Assignment to your teacher.
 Reflect and Connect
Reflect and Connect
RC 1. In the Get Focused section at the beginning of this lesson, three commonly used metals were identified: copper, silver, and gold. Examine the position of these metals on the large table of half-reactions shown in your Chemistry Data Booklet. How do the locations of these metals in the table correlate with the observations made in the Get Focused section?
Save your response in your course folder.
 Module 3: Lesson 2 Assignment
Module 3: Lesson 2 Assignment
Make sure you have completed both parts of the Lesson 2 Assignment:
- Part 1: Lab—Spontaneity of Redox Reactions
- Part 2: Lab Exercise 13.A on page 572 of the textbook
Submit your completed Module 3: Lesson 2 Assignment to your teacher.
1.13. Page 5
Module 3—Electrochemical Reactions
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following questions:
- Why do some metals appear to react more easily than other metals?
- Is it possible to predict whether an electrochemical reaction will occur spontaneously?
In this lesson you analyzed data collected from the reaction of metals with solutions of metal ions, and you developed a list ranking metals and metal ions in terms of their tendency to react. You also learned how to classify substances as oxidizing and reducing agents, and you learned how to rank them in terms of their tendency to transfer electrons. You learned about the spontaneity rule and how to use a table of redox reactions to predict the outcome of electrochemical reactions.
Lesson Glossary
non-spontaneous: a process that is incapable of proceeding unless driven by an outside source of energy
spontaneous: a process that occurs without any external energy source
A spontaneous process will occur on its own.
1.14. Lesson 3
Module 3—Electrochemical Reactions
Lesson 3—Half-Reactions
 Get Focused
Get Focused

© Christian Lagerek/shutterstock
Electrochemical reactions involve a transfer of electrons between species. So far in this module you have learned to identify oxidation, reduction, and agents that bring about the oxidation and reduction of other substances. In Lesson 2 you wrote half-reactions for simple changes to the charge of metals and metal ions. You also used empirical evidence, collected as you completed the investigation “Spontaneity of Redox Reactions,” to confirm the half-reactions you prepared.
You also began using the half-reactions listed on the “Table of Selected Standard Electrode Potentials” on page 7 of the Chemistry Data Booklet. Did you look at some of the other reactions in the table? If so, you may have noticed that many of the half-reactions involve more than one species.
Understanding how substances act alone or in combinations to bring about reactions in a chemical system is an important part of designing chemical systems to achieve a desired function. Chemists and technologists are required to investigate the behaviour of materials to ensure the materials will not undergo unwanted side reactions.
In some situations unexpected side reactions can occur when air, water, or other substances are present in a system. If their presence was not expected, an electrochemical reaction could result in the metal's corrosion, which could lead to metal fatigue and/or to a change in the metal's ability to function as intended.
Consider the following questions as you complete Lesson 3:
- How can combinations of species act together as oxidizing or reducing agents?
- Can half-reactions be used to predict and explain changes that occur within a chemical system?
- Can the same substance be the oxidizing agent and the reducing agent in an electrochemical process?
 Module 3: Lesson 3 Assignment
Module 3: Lesson 3 Assignment
Download a copy of the Module 3: Lesson 3 Assignment to your computer now. In this assignment you will develop an experimental design and complete the data collection and analysis for the lab “Testing Predictions.” You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.15. Page 2
Module 3—Electrochemical Reactions
 Read
Read
In Lesson 1 you learned that balancing chemical reactions involves ensuring that charge is balanced. When writing half-reactions, you will have checked that the net charge on each side of the equation was equal. In more complex half-reactions, balancing charge requires more care.
Read the section “Writing Complex Half-Reaction Equations” and work through the “Sample problems” and “Communication examples” on pages 564–567 of the textbook. You may wish to place a copy of “Summary” on page 567 in your course folder to review later in this module.
 Self-Check
Self-Check
SC 1. Complete “Practice” question 12 on page 566 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 12.
a.
Step 1: N2O(g) → N2(g)
Step 2: N2O(g) → N2(g) Nitrogen is balanced
Step 3: N2O(g) → N2(g) + H2O(l)
Step 4: 2 H+(aq) + N2O(g) → N2(g) + H2O(l)
Step 5: 2+ 0 = 0 +0
2+ = 0
Add e– to the most positive side.
![]()
b.
Step 1 & 2: NO2-(aq) → NO3-(aq)
Step 3: H2O(l) + NO2–(aq) → NO3–(aq)
Step 4: H2O(l) + NO2–(aq) → NO3–(aq) + 2 H+(aq)
0 -1 = -1 +2
-1 = +1
Step 5: H2O(l) + NO2–(aq) → NO3–(aq) + 2 H+(aq) + 2 e–
Step 6: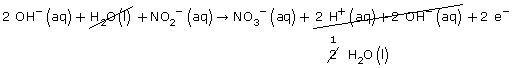
![]() (oxidation)
(oxidation)
c.
Step 1: Ag2O(s) → Ag(s)
Step 2: Ag2O(s) → 2 Ag(s)
Step 3: Ag2O(s) → 2 Ag(s) + H2O(l)
Step 4: 2 H+(aq) + Ag2O(s) → 2 Ag(s) + H2O(l)
Step 5: 2e– + 2 H+(aq) + Ag2O(s) → 2 Ag(s) + H2O(l)
Step 6: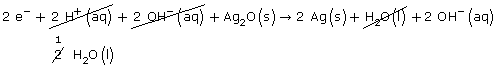
Step 7: ![]()
d.
Step 1 & 2: NO3–(g) → HNO2(aq)
Step 3: NO3–(g) → HNO2(aq) + H2O(l)
Step 4: 3 H+(aq) + NO3–(g) → HNO2(aq) + H2O(l)
Step 5: ![]()
e.
Step 1 & 2: H2(g) → H2O(l)
Step 3: H2O(l) + H2(g) → H2O(l)
Step 4: H2O(l) + H2(g) → H2O(l) + 2 H+(aq)
Step 5: H2O(l) + H2(g) → H2O(l) + 2 H+(aq) + 2 e–
Step 6 & 7: 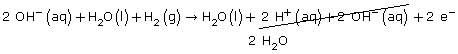
Simplifies:![]()
![]()
 Read
Read
When selecting materials for use in devices, it is useful to know what substances are present in the environment in which the device will be used. For example, if design manufacturers are considering using metals in machinery that will be exposed to water and oxygen, they need to know that those conditions may promote corrosion of the metal.
Look at the list of half-reactions in your Chemistry Data Booklet. Can you spot the half-reactions for iron and other metals? Can you locate the half-reaction for oxygen in combination with water? Is the position of the half-reaction containing oxygen and water acting as an oxidizing agent in a position that would result in a spontaneous reaction with iron?
Read page 575 to the end of “Communication example 1” on page 577 of the textbook. A summary of the method described in this section appears on page 578.
 Self-Check
Self-Check
SC 2. Complete “Practice” question 26 on page 579 of the textbook.
1.16. Page 3
Module 3—Electrochemical Reactions
 Lesson 3 Lab: Testing Predictions
Lesson 3 Lab: Testing Predictions
Background Information
In this lesson you learned to predict redox reactions using the five-step method listed on page 578 of the textbook. Are the predictions made using this method supported by empirical evidence? Having a method to predict and explain changes in metals, metal ions, or other substances in chemical systems would have great value when considering chemical systems that might exist beyond the ones you construct in a laboratory.
For instance, consider the equipment used in the extraction of bitumen in Alberta's oil sands operation. Electrochemical changes may be suspected in cases of metal fatigue in the equipment. Could the five-step method be used to identify alternative metals for use in this equipment?
In the Pre-Lab for this investigation you will design an experiment to test predictions using the following combinations of reactants:
System |
Reactant 1 |
Reactant 2 |
1
|
HCl(aq) |
Cu(s) |
2
|
HCl(aq) |
Mg(s) |
3
|
Cu(NO3)2(aq) |
Sn(s) |
4 |
NaOH(aq) |
I2(aq)
|
5 |
Cu(NO3)2(aq) |
Mg(s)
|
As you design your experiment, make sure you consider safety aspects when suggesting how these substances should be manipulated and how waste from the reactions should be disposed of.
As you saw in SC 2, it is important that you be able to use diagnostic tests, detect colour changes, measure changes to pH, or identify other evidence of reaction. The evidence of reaction that you predict and make certain you observe will allow you to form a conclusion about the method you are testing in this investigation.
![]() Retrieve the Module 3: Lesson 3 Assignment that you saved to your compluter earlier in this lesson. Complete the Pre-Lab section of the Assignment now. You will record your observations in your Assignment document as you view the virtual investigation, and then you will complete the Analysis section.
Retrieve the Module 3: Lesson 3 Assignment that you saved to your compluter earlier in this lesson. Complete the Pre-Lab section of the Assignment now. You will record your observations in your Assignment document as you view the virtual investigation, and then you will complete the Analysis section.
![]() View the virtual investigation “Testing Predictions for Redox Reactions.”
View the virtual investigation “Testing Predictions for Redox Reactions.”
 Module 3: Lesson 3 Assignment
Module 3: Lesson 3 Assignment
If you have not already done so, retrieve the Module 3: Lesson 3 Assignment that you saved to your compluter earlier in this lesson. Complete the Assignment. Save your work in your course folder. You will receive instructions later in this lesson on when to submit your Assignment to your teacher.
1.17. Page 4
Module 3—Electrochemical Reactions
 Read
Read
Some substances are not stable. One way to explain that lack of stability is to describe a reaction in which the same species acts as both the oxidizing and the reducing agent. Read “Disproportionation” on page 577–578 of the textbook.
 Read
Read
In this lesson you have learned how to
- use a redox table to predict the spontenaeity of reactions
- write half-reactions for species not on a redox table
Is it possible to add any oxidation and reduction half-reactions to produce a net ionic equation? Read “Predicting Redox Reactions by Constructing Half-Reactions” on pages 579–581 in the textbook. You will note that the method is very similar to the method you have already learned.
So far in this module, you have learned many skills for writing half-reactions and net ionic chemical equations, and for predicting whether spontaneous chemical change will occur when reactants are combined. You have also learned how to write chemical equations for substances that are not listed on the “Table of Selected Standard Electrode Potentials” in your Chemistry Data Booklet. If you write reactions that do not appear on this table, what aspect of this chemical system are you unable to determine?
 Self-Check
Self-Check
SC 3. Complete “Practice” question 31 on page 581 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Practice 31.
a.
4 [Zn(s) → Zn2+(aq) + 2 e-]
NO3-(aq) + 10 H+(aq) + 8 e- → NH4+(aq) + 3 H2O(l)
4 Zn(s) + NO3-(aq) + 10 H+(aq) → 4 Zn2+(aq) + NH4+(aq) + 3 H2O(l)
b.
Cl2(g) + 2 e- → 2 Cl-(aq)
SO2(g) + 2 H2O(l) → SO42-(aq) + 4 H+(aq) + 2 e−
Cl2(g) + SO2(g) + 2 H2O(l) → 2 Cl−(aq) + SO42-(aq) + 4 H+(aq)
 Reflect on the Big Picture
Reflect on the Big Picture

Early European explorers were surprised to find that the Inuit of northwestern Greenland were hunting with iron weapons despite the fact that the Inuit did not appear to have the technology to separate iron from its ore. To add to the puzzle, at the time, Greenland had no known iron ore deposits. Essentially, the Iron Age had begun for the First Nations people of northwestern Greenland. This was interesting because the use of iron was not documented anywhere else in the western hemisphere until contact was made with Europeans.
The mystery of how the Inuit had access to iron was solved when the explorers discovered that the Inuit were harvesting the iron from several huge meteorites. The Ahnighito meteorite pictured is 31 tonnes and is the largest fragment of a 59-tonne mass called the Cape York meteorite, which is believed to be the center of an early planet. The Ahnighito meteorite and some of the other, smaller fragments are on display in the American Museum of Natural History in New York City.
Iron found in ore deposits on Earth exists in an oxidized state, for example, as Fe2O3. Much of the iron found in a meteorite is in the form of the element, Fe. The Inuit were able to make tools using pieces of the Cape York meteorite because the pieces contain over 90% iron. Since meteorites form in space, many meteorites contain unique minerals (iron compounds) not normally found on Earth.
One of the most studied meteorites to strike Canada is the Abee meteorite, which struck Earth in 1952 near Abee, Alberta. The Abee meteorite is only 32% iron. Accounts indicate that the Abee meteorite smells like gunpowder due to the large proportion of sulfur it contains. The unique iron and sulfur minerals in this meteorite, such as djerfisherite (K3CuFe12S14), may suggest that the meteorite was once a piece of the planet Mercury and was formed over 4.5 billion years ago. The meteorite was extracted from a two-meter-deep hole in a wheat field by a farmer, a school teacher, and several other interested townsfolk. The Abee meteorite is currently part of the National Meteorite Collection and remains available for further study.

When Chemistry 30 Learn EveryWare was being developed, a meteorite was observed in the night skies over Alberta. Pieces were found near Lone Rock, Saskatchewan. You might research the composition of this meteorite and the theories about its origin.
RBP 1. Use your knowledge of electrochemistry to explain how it is possible for iron to exist in different forms (both as a pure substance and within compounds) found in Earth and in space as evidenced by the composition of the Cape York and Abee meteorites.
Save a copy of your response in your course folder.
 Module 3: Lesson 3 Assignment
Module 3: Lesson 3 Assignment
Submit your completed Module 3: Lesson 3 Assignment to your teacher.
1.18. Page 5
Module 3—Electrochemical Reactions
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- How can combinations of species act together as oxidizing or reducing agents?
- Can half-reactions be used to predict and explain changes that occur within a chemical system?
- Can the same substance be the oxidizing agent and the reducing agent in an electrochemical process?
Redox reactions can involve a variety of substances, which sometimes act in combination to promote the oxidation or reduction of other substances. You learned that half-reactions can be used to describe the chemical changes that occur within a system. If provided with a table of half-reactions written as reductions, you are able to predict which combinations of substances will react spontaneously.
1.19. Lesson 4
Module 3—Electrochemical Reactions
Lesson 4—Oxidation Numbers and Corrosion Protection
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
Throughout this module you have considered the reaction of metals with other substances. You have learned many techniques for interpreting the changes observed in chemical systems undergoing electrochemical change. You have also learned many new skills, namely the ability to predict the spontaneity of reactions and to explain disproportionation reactions.
Many structures rely on iron for their integrity. For example, reinforced concrete contains rods of steel, which is primarily composed of iron, called rebar. The frames of automobiles also rely on iron for their structural integrity. But we know iron can rust and, when it does, it loses its integrity. In the Module 3 Big Picture you read about the restoration of the High Level Bridge in Edmonton, Alberta, and the quantity of corroded material that was removed from the bridge. Since so many materials rely on iron or steel, corrosion has a great economic impact and presents serious safety concerns.
In Lesson 4 you will investigate the process of corrosion and how it can be further understood as a redox process. You will also learn about methods that are used to prevent the corrosion of iron.
Consider the following questions as you complete Lesson 4:
- What are oxidation numbers and how can they be used to understand redox reactions?
- What factors cause corrosion?
- How can corrosion be prevented?
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
Download a copy of the Module 3: Lesson 4 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. The assignment has the following three parts:
- Part 1: Lab—Oxidation States of Manganese
- Part 2: Lab—Corrosion of Nails
- Part 3: Questions
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.20. Page 2
Module 3—Electrochemical Reactions
 Explore
Explore
 Try This
Try This
Iron Corrosion
The corrosion of iron is a complex electrochemical process. Steel is primarily composed of iron. Its corrosion occurs more frequently in moist conditions in which the iron is also exposed to oxygen.
The process of corrosion involves more than a simple oxidation of iron to Fe2+. Iron(II) ions further undergo a reaction to form rust, which has the chemical formula Fe2O3 • nH2O(s).
TR 1. Identify the strongest oxidizing and reducing agent in the initial reaction of iron described in the first paragraph of the passage above.
TR 2. Identify whether the change in iron that forms rust, described in the second paragraph, is an oxidation or a reduction. Support your answer.
Submit your answers to your teacher for feedback. Save a copy of your answers in your course folder, as you may wish to refer to them later in this lesson.
 Read
Read
How did you determine that iron was undergoing a change in TR 1 and TR 2? Did you focus on the charge on the iron atom? For metals, determining whether a reduction or an oxidation has occurred often requires you to look at how the charge of the metals changes. What about non-metals and atoms within polyatomic ions? How is their oxidation or reduction tracked?
Read pages 583–585 in the textbook. You may wish to save a copy of the information in “Table 1” on page 583 in your course folder.
Earlier in this module you compared the charge of copper atoms and copper ions. When doing so, you were using the oxidation numbers for these forms of the copper atom. Using oxidation numbers is a quick and useful way to obtain information about electrochemical change. Since oxidation numbers are a powerful tool in analysis of electrochemical systems and you will not always be able to access this information in your Chemistry Data Booklet, you will have to memorize the information in this table.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–5 on page 585 of the textbook.
 Read
Read
At the beginning of this lesson you were asked to consider how the electrochemical change in atoms could be tracked. Can oxidation numbers be used to identify the oxidized and reduced species (reducing agent and oxidizing agent respectively) in a balanced chemical equation? Read the text and work through the “Sample problems” and “Communication examples” on pages 585–588 of the textbook to find out.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 6–9 on page 588–589 of the textbook.
1.21. Page 3
Module 3—Electrochemical Reactions
 Lesson 4 Lab: Oxidation States of Manganese
Lesson 4 Lab: Oxidation States of Manganese
In this investigation you will use your skills of observation to investigate the changes in oxidation number that can occur with certain transitional metals. In preparation for the investigation, complete SC 3.
 Self-Check
Self-Check
SC 3. Complete “Lab Exercise 13.B” and “Analysis” questions a–c on page 586 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
a.
Reaction in Table 3 |
Vanadium Ions in Sequence of Reactions |
1 |
VO3–(aq) |
2 |
VO3–(aq) → VO2+(aq) → V3+(aq) → V2+(aq) |
3 |
V2+(aq) → V3+(aq) |
4 |
VO3–(aq) → VO2+(aq) |
5 |
VO2+(aq) (no change) |
6 |
V2+(aq) → V3+(aq) → VO2+(aq) → VO3–(aq) |
b.
Reaction in Table 3 |
Description of Chemical Change |
1 |
Ammonium vanadate(V) dissociates, thus no chemical change or change in oxidation number occurs. |
2 |
Vanadium is reduced from +5 to +2. |
3 |
Vanadium is oxidized from +2 to +3. |
4 |
Vanadium is reduced from +5 to +4. |
5 |
There is no change in oxidation number. |
6 |
Vanadium is oxidized from +2 to +5. |
c.
Reaction in Table 3 |
Explanation of Chemical Change |
3 |
Vanadium is oxidized from +2 to +3, probably due to exposure to atmospheric oxygen. |
4 |
Vanadium is reduced from +5 to +4, forming VO2+(aq). The blue colour of the VO2+(aq) coincides with the appearance of aqueous iodine, the product of the other half-reaction in the system. Aqueous iodine is brown in colour. The combination of the two colours results in the solution appearing black. |
5 |
There is no change in oxidation number because iodide ions do not react spontaneously with VO2+(aq). |
6 |
Vanadium is oxidized from +2 to +5 forming VO3-(aq), since permanganate ions in acidic conditions react spontaneously with V2+(aq). |
![]() Retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. You will complete Part 1 as you view the virtual investigation “Oxidation States of Manganese.”
Retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. You will complete Part 1 as you view the virtual investigation “Oxidation States of Manganese.”
![]() View the virtual investigation “Lesson 4 Lab: Oxidation States of Manganese.” Remember to record data and observations as you view the presentation.
View the virtual investigation “Lesson 4 Lab: Oxidation States of Manganese.” Remember to record data and observations as you view the presentation.
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
If you have not already done so, retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete Part 1. You will receive instructions later in the lesson on when to complete the rest of the Lesson 4 Assignment and submit your work to your teacher.
1.22. Page 4
Module 3—Electrochemical Reactions
 Read
Read
To this point you have used oxidation numbers to identify oxidized and reduced species. Read the text and work through the “Sample problems” and “Communication examples” on pages 589–593 in the textbook to see how using oxidation numbers can enable you to balance entire redox reactions.
Mastering this method will add to your skills and may save you time in completing problems in the future. You may recall that, in Lesson 3, the method to balance half-reactions and to write a net ionic equation had many steps. You may find this method preferable as an alternative or as a means to check work in which you used the other methods of writing reactions.
 Self-Check
Self-Check
SC 4. Complete “Practice” question 12 on page 593 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 4.
Practice 12.
a.
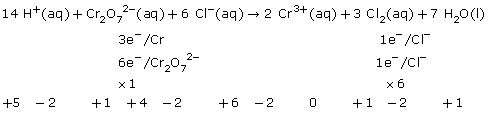
b.
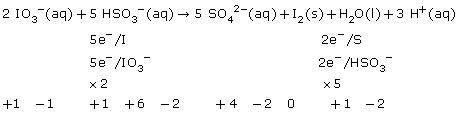
c.
After balancing a net ionic equation using oxidation numbers, you should check to see if the charge on each side balances.
1.23. Page 5
Module 3—Electrochemical Reactions
 Lesson 4 Lab: Conditions Affecting the Corrosion of Iron
Lesson 4 Lab: Conditions Affecting the Corrosion of Iron
Problem
Do various conditions influence the corrosion of iron?
Purpose
To investigate conditions that influence the corrosion of iron objects and to determine if the chemical change observed can be explained using concepts discussed in this module.
![]() Retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 as you work through the following investigation. When you have finished, save your completed Assignment in your course folder. You will receive instructions later on when to complete the rest of this Assignment.
Retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 as you work through the following investigation. When you have finished, save your completed Assignment in your course folder. You will receive instructions later on when to complete the rest of this Assignment.
Materials
- steel wool
- acetone
- 100-mL beaker
- six nails
- six 13 mm × 100 mm test tubes
- two rubber stoppers
- test tube rack
- NaOH(aq)
- HCl(aq)
- NaCl(aq)
- deionized (distilled) water
- deaerated water
- safety glasses
- lab apron
If you have access to the materials listed, you may be able to perform this investigation.
![]() If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 4 Lab: Conditions Affecting the Corrosion of Iron.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 4 Lab: Conditions Affecting the Corrosion of Iron.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
Procedure
Step 1: Measure 25 mL of acetone.
Step 2: Clean six nails with steel wool, and place the nails in the acetone. Use forceps to remove the nails and then set the nails on a paper towel to dry.
Step 3: Set six 13 mm × 100 mm test tubes in a test tube rack.
Step 4: Into five of the test tubes, transfer approximately 6 mL of the following solutions: distilled water, HCl(aq), NaCl(aq), NaOH(aq), and deaerated water. Label each test tube with the name of its contents.
Step 5: Label the sixth test tube “air.”
Step 6: Place a clean, dry nail in each test tube, and place a stopper in tubes 5 (dearerated water) and 6 (air).
Step 7: Set the rack containing the test tubes and nails aside for 24 hours.
Step 8: Observe the results, including solution colour, nail colour, and nail integrity. Record your observation in the data section in the Module 3: Lesson 4 Assignment.
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
If you have not already done so, retrieve your copy of the Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete Part 2. You will receive information later in the lesson on when to complete Part 3 of the Assignment and when to submit your work to your teacher.
1.24. Page 6
Module 3—Electrochemical Reactions
 Reflect and Connect
Reflect and Connect
Billions of dollars are spent every year to prevent or slow the corrosion of iron. The following are some methods to prevent the corrosion of iron.
- Mix metals with iron to form a more corrosion-resistant alloy. Alloys have a unique set of properties. Stainless steel is an iron alloy. It contains mostly iron, between 10% to 30% chromium, a smaller percentage of nickel, and sometimes cobalt.
- Paint exposed iron surfaces. Applying a coating prevents water and oxygen from gaining access to iron and therefore prevents oxidation of the iron.
- Plate iron with another metal. Like paint, the layer of other metal prevents water and oxygen from gaining access to iron. Chromium and zinc (galvanization) are commonly used for plating iron. You will learn more about electroplating in the next module.
- Apply oils and grease to exposed iron. This method is often used on the moving parts of machinery. Oils and grease repel water because of the lack of polarity in the molecules.
You will address a corrosion problem in the Module 3 Assessment. You might want to view the Module 3 Assessment now and consider which of the four methods listed above might be applied to the corrosion problem. You may wish to record any ideas you have so that you can revisit them after you have completed all the lessons in this module and are ready to complete the Module 3 Assessment.
You will learn more about other methods to protect iron from corrosion in Module 4.
 Module 3: Lesson 4 Assignment
Module 3: Lesson 4 Assignment
Retrieve your copy of Module 3: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete Part 3: Questions. Save your work in your course folder.
Make sure you have completed all parts of the Assignment:
- Part 1: Lab—Oxidation States of Manganese
- Part 2: Lab—Corrosion of Nails
- Part 3: Questions
Submit your completed Module 3: Lesson 4 Assignment to your teacher.
1.25. Page 7
Module 3—Electrochemical Reactions
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following questions:
- What are oxidations numbers and how can they be used to understand redox reactions?
- What factors cause corrosion?
- How can corrosion be prevented?
Corrosion is a complex process involving more than one redox reaction. You must pay careful attention to the charge on the iron ion in order to understand the steps in the corrosion process. For other species, oxidation numbers provide insight into the chemical change of the species and the involvement of redox processes.
Lesson Glossary
alloy: a homogeneous mixture of two or more metals or of a metal and a metalloid
1.26. Lesson 5
Module 3—Electrochemical Reactions
Lesson 5—Redox Stoichiometry
 Get Focused
Get Focused

The label on this water treatment system says “IRON Eater.”
In previous chemistry courses you learned about quantitative analysis—the determination of the quantity of substance present in a chemical system. Quantitative analysis is important in all branches of chemistry, design, and engineering.
Earlier you learned that 45 tonnes of rust were removed in the restoration of Edmonton’s High Level Bridge. Now that you know more about the process of corrosion, you might ask yourself how many tonnes of iron were oxidized and, therefore, what quantity of metal needed to be replaced?
Many domestic systems also involve redox reactions. One of these is iron filtration, a commonly used water treatment method for people who rely on water from a well. In Lesson 5 you will learn about the design and use of iron filtration. You will also further your understanding of stoichiometry in a quantitative analysis of chemical systems involving redox reactions.
Consider the following questions as you complete Lesson 5:
- How can a chemical system be analyzed using redox reactions?
- How is the stoichiometric method applied to redox systems?
 Module 3: Lesson 5 Assignment
Module 3: Lesson 5 Assignment
Download a copy of the Module 3: Lesson 5 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. The assignment has the following parts:
- Part 1: Lab—Analyzing a Hydrogen Peroxide Solution
- Part 2: Reflect and Connect
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.27. Page 2
Module 3—Electrochemical Reactions
 Explore
Explore

Corrosion is not the only problem iron presents. Iron is one of the most plentiful elements in Earth’s crust; as such, iron can be present in groundwater. In Alberta and elsewhere, iron can be a problem in well water. Iron(II) ions give well water a pale yellow-brown colour, and iron(III) ions can create insoluble precipitates. Iron in water also gives water a metallic taste and can stain dishes and laundry.
Many people using well water install water treatment systems to remove iron from their water. A popular method to remove iron from water involves treating iron with potassium permanganate, KMnO4(s). How might this system be able to remove iron(II) ions from well water?
You may recall that permanganate ion is a strong oxidizing agent. When potassium permanganate is pumped into the filter, it brings about the oxidation of iron(II) ions to iron(III) ions. Iron(III) ions are poorly soluble in water and precipitate. The precipitate is then removed by the filter.
 Self-Check
Self-Check
SC 1. Write the half-reactions and balanced net ionic equation for the reaction between potassium permanganate and iron(II) ions that occurs in the iron filter system. Assume acidic conditions.
SC 2. Is the reaction spontaneous?
SC 3. Potassium permanganate in an aqueous solution at low concentrations has an intense purple colour. How would a consumer know if the quantity of potassium permanganate used in the iron filter was sufficient?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
MnO4-(aq) + 8 H+(aq) + 5 e- → Mn2+(aq) + 4 H2O(l)
5 [Fe2+(aq) → Fe3+(aq) + 1 e-]
5 Fe2+(aq) + MnO4-(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
SC 2. The reaction is spontaneous.
SC 3. When permanganate reacts, it undergoes reduction to form Mn2+(aq). The manganese(II) ion at low concentrations is colourless. If appropriate quantities of permanganate are being used, the water from the iron filter will appear colourless. If excessive permanganate is used, the water will appear pink or purple in colour. If not enough permanganate is used, the water will appear pale yellow-orange in colour due to the presence of iron(II) ions.
 Read
Read
The iron filtration system demonstrates the application of a redox reaction in a water treatment system. It also demonstrates how sensitive some processes are to the quantities of substances used.
In previous chemistry courses you studied the stoichiometric method and performed a titration experiment. Can you think of how this water treatment system is similar to a titration and a stoichiometric relationship?
Read “Titration” on page 804, and read all of page 596 of the textbook.
 Self-Check
Self-Check
SC 4. In previous chemistry courses you performed titrations in acid-base systems. You used pH indicators to detect the endpoint of the titration.
Explain the following aspects of a titration:
- What unique glassware is used in a titration and what is the function of each piece?
- What is an endpoint?
- What property of a potassium permanganate solution could be used as an endpoint for a titration?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 4.
a. A burette (to measure volume of titrant added to reach endpoint) and a pipette (to deliver a specific volume of solution of unknown concentration (test solution)) are used in a titration.
b. The endpoint is a point in a titration when an observable change in the system occurs. Usually this change is a colour change, but it can be another change such as a change in pH or conductivity.
c. Colour could be used as an endpoint. The persistence of the purple MnO4-(aq) indicates an excess of permanganate ion in the solution. Permanganate ion can only exist in excess in the solution once all of the reactant in the test solution has reacted.
 Read
Read
You will recall from your work in previous science courses that a titration involves adding a solution with a known concentration to a test solution with an unknown concentration. When you performed a titration experiment previously, you made careful observations and you recorded the volume of each solution involved in the reaction. Using the information about the volumes in addition to the molar concentration of the titrant allows for calculation of the chemical quantities involved. You will also recall that knowledge of the balanced chemical equation for the reaction occurring in the titration is critical to performing any titration analysis.
Given the importance of writing redox reactions to performing quantitative analysis, you may understand why so much emphasis was placed on learning how to write balanced chemical equations for redox reactions earlier in this module.
To complete your work in this lesson you will need to join the following two sets of skills:
- writing redox reactions
- performing stoichiometric calculations using titration data
Carefully read and work through “Sample problem 13.14” on page 597 of the textbook to learn how to join these skills.
 Self-Check
Self-Check
SC 5. Complete “Practice” questions 3 and 5 on page 598 of the textbook.
1.28. Page 3
Module 3—Electrochemical Reactions
 Lesson 5 Lab: Analyzing a Hydrogen Peroxide Solution
Lesson 5 Lab: Analyzing a Hydrogen Peroxide Solution
In this investigation you will perform a titration analysis to determine the concentration of hydrogen peroxide in a commercially sold preparation.
![]() Retrieve your copy of the Module 3: Lesson 5 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 5 Assignment.
Retrieve your copy of the Module 3: Lesson 5 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 5 Assignment.
Read page 603 in the textbook to familiarize yourself with the purpose, problem, design, materials, and procedure you will follow. Then, complete the lab as described on page 603 of the textbook.
![]() If you do not have access to the materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 5 Lab: Analyzing a Hydrogen Peroxide Solution.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
If you do not have access to the materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 5 Lab: Analyzing a Hydrogen Peroxide Solution.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
 Module 3: Lesson 5 Assignment
Module 3: Lesson 5 Assignment
Retrieve your copy of the Module 3: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete Part 1 of the Assignment. Save your completed Assignment in your course folder. You will receive instructions later in this lesson on when to complete Part 2 and when to submit your work to your teacher.
1.29. Page 4
Module 3—Electrochemical Reactions
 Reflect and Connect
Reflect and Connect
Earlier in this lesson you learned about the use of potassium permanganate in an iron filtration system for well water.
![]() Retrieve your copy of the Module 3: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 of the Assignment. Save your completed Assignment in your course folder and submit a copy to your teacher.
Retrieve your copy of the Module 3: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 of the Assignment. Save your completed Assignment in your course folder and submit a copy to your teacher.
1.30. Page 5
Module 3—Electrochemical Reactions
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following questions:
- How can a chemical system be analyzed using redox reactions?
- How is the stoichiometric method applied to redox systems?
An iron filtration system uses a strong oxidizing agent, permanganate ions, to oxidize iron(II) ions into a form that allows them to be filtered and removed from drinking water. The selection of permanganate as a reactant not only assures a spontaneous and stoichiometric reaction but also allows for assessment of the system's operation.
Similar principles are applied in the design of a redox titration in which spontaneous and stoichiometric reactions are used to perform quantitative analysis. Some oxidizing agents have distinctive colours, like permanganate and dichromate. As these substances undergo electrochemical change, their colour change or lack of change can provide a detectable endpoint for titrations, allowing for accurate quantitative analysis.
1.31. Module Summary/Assessment
Module 3—Electrochemical Reactions
 Module Summary
Module Summary
In Module 3 you considered the following module questions:
- What properties of metals make them popular choices in the construction and production of materials?
- How can an understanding of corrosion allow for better selection of materials and for development of methods that reduce material damage?
Your study of electrochemical change in Module 3 has prepared you to understand the rationale for many technologies used in products you will purchase. You also now understand the importance of maintaining or improving the methods used to protect metals from conditions in which the metals may react and fail to perform as intended.
Concept Map or Graphic Organizer
As you worked through Module 3, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 3 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 3 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
Consider these photographs:

- Describe the electrochemical change that has discoloured the iron used to construct the granary. Your description must mention oxidation numbers and include a relevant half-reaction.
- List technologies that could have been used to prevent this change from occurring. Select one of the technologies you listed, and prepare a detailed description of how this method prevents this type of change.
1.32. Module Glossary
Module 3—Electrochemical Reactions
Module Glossary
alloy: a homogeneous mixture of two or more metals or a metal and a metalloid
non-spontaneous: a process that is incapable of proceeding unless driven by an outside source of energy
spontaneous: a process that occurs without any external energy source
A spontaneous process will occur on its own.