Module 4
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 4 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 11:14 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 4
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Page 6
- 1.9. Lesson 2
- 1.10. Page 2
- 1.11. Page 3
- 1.12. Page 4
- 1.13. Page 5
- 1.14. Page 6
- 1.15. Page 7
- 1.16. Lesson 3
- 1.17. Page 2
- 1.18. Page 3
- 1.19. Page 4
- 1.20. Lesson 4
- 1.21. Page 2
- 1.22. Page 3
- 1.23. Page 4
- 1.24. Lesson 5
- 1.25. Page 2
- 1.26. Page 3
- 1.27. Page 4
- 1.28. Module Summary/Assessment
1. Module 4
Module 4—Batteries and Balance
Module Introduction

© Adam Majchrzak/shutterstock
When you look at this picture, do you see many different kinds of batteries or do you see many different shapes of the same chemical system? Your answer might indicate whether you view batteries from a technological perspective or from a scientific perspective.
In this module you will extend and apply your understanding of electrochemical change to understanding the design and function of a variety of types of electrochemical cells, one of which is the electric cell—the technology used in batteries and in other applications.
You will use the skills and techniques you have been developing to analyze and predict change in electrochemical systems. You will do this within the context of electric and electrolytic cells. You will also investigate the technological aspects of, and your reliance on, these chemical systems.
As you investigate the electrochemical cells introduced in this module, you will see how chemical knowledge about reduction-oxidation reactions is applied to solving problems and expanding capabilities. You will consider the purpose and impact of different types of electrochemical cells.
In Module 4 you will investigate the following question:
- How are principles of oxidation and reduction applied to electrochemical cells?
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 4, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit B Concept Organizer. In the Module 4 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 4—Batteries and Balance
 Big Picture
Big Picture

© 2008 Jupiterimages Corporation
It’s the end of a long day. Your end-of-day routine probably includes checking your cell phone, your MP3 player, and a few other devices to see if their batteries need to be recharged. You probably haven’t considered all the other devices in your home that rely on batteries in order to work.

The batteries in a laptop computer or in a cordless telephone provide you with great convenience, but at what cost? While some batteries are rechargeable, others are not. Every battery has a certain lifespan. When a battery reaches the end of its lifespan, it is usually just thrown away without further thought. However, batteries that end up in landfills can leak toxic chemicals into the environment.
If you ever make a trip to the Eco Station or special waste handling facility in your area, you might be amazed by how many electronic devices are disposed of. What about the batteries that powered these devices? Is it time to think about your use of batteries and to make some changes in how you use them?
In Module 4 you will learn about the design and operation of electrochemical cells. You will apply your understanding of electrochemical change learned in Module 3. You will continue to use tools like the “Table of Selected Standard Electrode Potentials,” your ability to predict spontaneous and non-spontaneous reduction-oxidation reactions, and stoichiometry throughout Module 4.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 4 Assessment you will apply your knowledge of electrochemical systems to investigate a cell constructed using a potato or a lemon.
You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 4—Batteries and Balance
In This Module
Lesson 1—Voltaic Cells and Batteries
In Lesson 1 you will learn about the scientific principles involved in the construction and function of voltaic cells, a type of commercial electric cell.
You will investigate the following lesson questions:
- How much do you rely on batteries in your everyday life?
- What are the components of a voltaic cell?
Lesson 2—Design of Commercial Cells
In Lesson 2 you will investigate the construction of different types of commercial and consumer cells used by society, and you will consider the environmental impact of your personal battery use.
You will investigate the following lesson question:
- Why are there so many different designs and types of electrochemical cells?
Lesson 3—Electrolytic Cells
Lessons 1 and 2 will explain the chemical change that occurs within a voltaic cell. But how can you make a cell rechargeable? In Lesson 3 you will learn about rechargeable cells and why some cells are rechargeable and others are not.
You will investigate the following lesson questions:
- What is an electrolytic cell?
- What chemical and energy conversions occur within an electrolytic cell?
Lesson 4—Commercial Applications of Electrolytic Cells
In Lesson 4 you will learn about industrial applications of electrolysis, and you will learn how this technology can be applied to the extraction of elements and to the refining and electroplating of metals.
You will investigate the following lesson question:
- What are some of the practical applications of electrolytic cells?
Lesson 5—Quantitative Relationships in Cells
In Lesson 5 you will record and analyze qualitative changes within electric and electrolytic cells.
You will investigate the following lesson question:
- What are the important quantitative relationships within operating electric and electrolytic cells?
1.3. Lesson 1
Module 4—Batteries and Balance
Lesson 1—Voltaic Cells and Batteries
 Get Focused
Get Focused

© Adam Majchrzak/shutterstock
The commercial “batteries” you use are made up of components involved in an electrochemical reaction. In Module 3 you learned about the scientific principles associated with electrochemical change. In this lesson you will begin to investigate how materials can be used to make model systems and commercial cells that allow for the study and use of the electron exchange that occurs between components.
You will start your investigation of electrochemical cells by looking at the most-used type of electric cell—the voltaic cell. By learning more about the voltaic cell, you might even be able to identify ways to improve this technology and its application.
Consider the following questions as you complete Lesson 1:
- How much do you rely on batteries in your everyday life?
- What are the components of a voltaic cell?
 Module 4: Lesson 1 Assignment
Module 4: Lesson 1 Assignment
There is no assignment for this lesson.
There are other questions in this lesson that are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 4—Batteries and Balance
 Explore
Explore
 Try This
Try This
Battery Audit
Perform an audit of the commercial electric cells, or “batteries,” used in your home. You might identify all of the devices powered by batteries that you or your family recently used, or you might count the number and types of cells you replaced recently.
TR 1. In a table or spreadsheet, list each type of cell and its quantity. You might also want to indicate what device the cell came from (e.g., alarm clock) and, if you can recall, the last time the cell was changed in the device. For devices that you place on a recharger (e.g., cell phone), you might want to indicate the components of the cell (e.g., lithium ion, nickel-cadmium, metal hydride) and how often you recharge the cell. Finally, include an estimate of the quantity of cells you would use in one year.
Save your work in your course folder.
 Discuss
Discuss
Place a copy of your audit in the discussion area for your class. Look at the totals submitted by other students. How would you rank your household’s use of batteries relative to that of other households?
 Try This
Try This
In previous chemistry courses you became aware of chemical waste, which is the product of chemical processes. In this module you will learn more about the contents of commercial cells and the products of the chemical reactions that occur within them. Now is a good time to think about the mass of chemical waste products that come from commercial cells.
| Cell Type | Mass (g) |
|---|---|
| AAA | 11.2 |
| AA | 23.9 |
| C | 70.0 |
| 9-volt | 46.6 |
| D | 149.9 |
| coin type | 3.1 |
TR 2. Prepare a spreadsheet to calculate the mass of each type of commercial electric cell that is disposed of by two of the following groups of people. You might use the totals in your audit or in the audits of your classmates to determine average use where required.
- your household
- your class
- your local community
TR 3. How does your household dispose of spent cells? Survey other students in your class to see if there are other ways to dispose of spent cells.
Save a copy of your answers in your course folder. You may be asked to submit a copy to your teacher for feedback.
 Read
Read
Electrochemical cells are devices built to use the transfer of electrons that drives redox reactions. The components of an electrochemical cell are the reactants for a reduction-oxidation process. The design allows the exchange of electrons between reactants to do work. Keep this principle in mind as you read pages 612–614 in the textbook.
1.5. Page 3
Module 4—Batteries and Balance
 Module 4: Lesson 1 Lab—The Voltaic Cell
Module 4: Lesson 1 Lab—The Voltaic Cell
Alessandro Volta (1745–1827) is credited with the discovery of the construction and design of the first electric cells. The name voltaic is often used to describe the type of cell you will study in this module. One type of voltaic cell design uses copper and zinc electrodes. This type of cell is often studied due to its reliability. In this investigation you will construct and test a voltaic cell.
Pre-Lab
Earlier in this lesson you learned that the principles of electrochemical reactions you investigated in Module 3 could be applied to understand the operation of the cells you will investigate in Module 4. Before you view the virtual investigation for this lab, complete Self-Check questions 1–7.
 Self-Check
Self-Check
Consider this diagram:
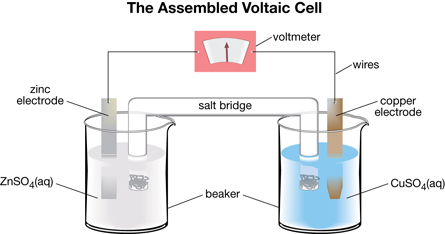
SC 1. List the two metals used in the cell. Use the “Table of Selected Standard Electrode Potentials” in your Chemistry Data Booklet to determine which one of the two metals is the more reactive metal. Repeat this step for the metal ions in the cell.
SC 2. Use the “Table of Selected Standard Electrode Potentials” in your Chemistry Data Booklet to determine the oxidation and reduction half-reactions that would occur in the voltaic cell illustrated.
SC 3. Use your knowledge of spontaneous reactions to determine whether the more reactive metal and metal ion will react spontaneously in the cell shown in the diagram.
SC 4. Use your half-reactions to determine which metal is losing electrons and which metal ion is gaining electrons.
SC 5. Use your spontaneous half-reactions to predict what will happen to each metal strip if you were to let the voltaic cell sit for a long time.
SC 6. If the voltaic cell kept operating indefinitely, would any part of the cell labelled in the diagram need to be replaced? Explain your reasoning.
SC 7. Hypothesize which metal strip is the negative electrode of the voltaic cell and which metal strip is the positive electrode of the voltaic cell. Provide a reason for your choice.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. Copper and zinc are used in the cell. Zinc is the more reactive metal. The copper ion is the more reactive metal ion.
SC 2. The redox half-reactions will involve the reduction of the most reactive metal ion and the oxidation of the most reactive metal.
reduction: Cu2+(aq) + 2e– → Cu(s)
oxidation: Zn(s) → Zn2+(aq) + 2e–
SC 3. The reaction will be spontaneous because the reduction half-reaction appears above the oxidation half-reaction in the "Table of Selected Standard Electrode Potentials." From experiments performed in Module 3, it is known that reactants with similar positions on the table react spontaneously.
SC 4. As stated in the half-reactions, the zinc will lose electrons and the copper ions will gain electrons.
SC 5. The zinc electrode should reduce in mass and possibly in size as the zinc atoms lose electrons and are changed into zinc ions that will go into solution. The copper electrode should increase in mass as the copper ions come out of the solution to join with electrons to form copper metal.
SC 6. A voltaic cell is a closed chemical system, and the reactants will be depleted as the reactions proceed. Unless more copper(II) ions are placed into the cell and more zinc is added, no reactants will be present to change electrons.
SC 7. Since the zinc metal is the site of oxidation and, therefore, the source of electrons, it is the negative electrode. The copper electrode is the destination for the electrons in contact with the copper ions that will gain the electrons. This makes the copper metal the positive electrode.
Procedure
![]() View the virtual investigation “Building a Voltaic Cell.” Remember to record your data and observations as you view the presentation.
View the virtual investigation “Building a Voltaic Cell.” Remember to record your data and observations as you view the presentation.
Analysis
Use your observations from the virtual investigation to complete Self-Check questions 8–11.
 Self-Check
Self-Check
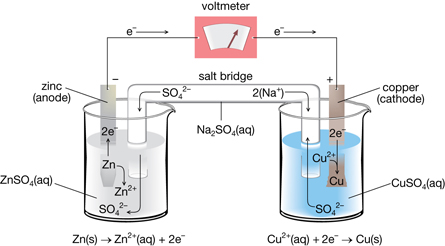
SC 8. Describe the effect on the output of the voltmeter when you lifted the salt bridge out of the cell.
SC 9. Hypothesize why the salt bridge is necessary for the voltaic cell to work.
SC 10. Explain what happened to the output of the voltmeter when you connected the leads from the voltmeter to the opposite electrodes in the cell.
SC 11. Describe the changes you observed to the electrodes or to the solutions. Use the half-reactions developed in SC 2 to help suggest reasons for the changes you observed.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 8. When the salt bridge was lifted out of the solution, the voltage dropped to zero.
SC 9. Hypothesis: Systems need to maintain a balance in their function. In this cell, electrons transfer between the parts of the cell. When electrons transfer, there is a flow of negative charge. To maintain a balance, there must also be a flow of positive charge to balance the flow of negative charge.
The salt bridge allows ions in the electrolytes to move, thus completing the flow of charge in the circuit. If electrons are flowing along the wire from one electrode to the other, there must be a movement of ions within the cell to complete the circuit. This is completed by the movement of dissociated ions in the solutions through the salt bridge.
SC 10. When the leads from the voltmeter were connected to the opposite electrodes in the voltaic cell, the voltmeter switched from displaying a positive output to displaying a negative output. If the original output was negative, it would have switched to positive. In both cases, the output changes because the electrons are flowing through the voltmeter in the opposite direction.
SC 11. The copper electrode had a reddish-brown sediment on its surface. This is the copper metal being deposited from the copper ions that come out of the solution as described by the reduction half-reaction. Since the copper ions are being removed from the solution containing the copper electrode, this solution appeared to have a slightly less intense colour after the cell had been operating. Although no visible changes could be observed on the zinc electrode, this electrode would diminish in mass and size if the voltaic cell were able to run for a long time.
1.6. Page 4
Module 4—Batteries and Balance
 Read
Read
In the investigation you just completed you identified the parts of a voltaic cell and, in combination with your pre-lab work, you identified reactions that involve different components of the cell. Read page 622 and the top of page 623 of the textbook to learn more about other necessary components of voltaic cells and about how voltaic cells are represented.
 Try This
Try This
TR 4. A silver-copper voltaic cell is constructed.
- Draw a diagram of the cell. Label the location of the following components: Ag(s), AgNO3(aq), Cu(s), Cu(NO3)2(aq), salt bridge, electrical wire.
- Write the reduction half-reaction occurring in this cell.
- Write the oxidation half-reaction occurring in the cell.
- Label the anode and cathode of the cell.
- Use an arrow to indicate the direction of the flow of electrons along the electrical wire.
TR 5. The salt bridge, or porous boundary, is an essential part of a voltaic cell. Since electrons are exchanged between the half-cells, ions must also be exchanged in order to conserve charge.
- Predict the direction of the flow for positively charged ions (cations) with respect to the cathode half-cell.
- Predict the direction of the flow for negatively charged ions (anions) with respect to the anode half-cell.
 Watch and Listen
Watch and Listen
The Voltaic Cell
View the animation “The Voltaic Cell.” Use the information in the animation to check your answers to questions TR 4 and TR 5. You may also wish to read pages 623–624 in the textbook for another description of the operation of, and changes occurring within, a silver-copper voltaic cell.
 Read
Read
Voltaic cells can also be constructed with half-cells that do not involve a metal as a reactant. An inert electrode can be used if a solution contains the strongest oxidizing agent or the strongest reducing agent in an aqueous form. The term inert means that the electrode cannot react during the cell’s operation. An electrode is only necessary to be an electrical conductor—to allow for the movement of electrons in a half-cell.
Read pages 625–626 in the textbook to learn how an inert electrode is used in a voltaic cell. Work through “Communication example 1” on page 625 to check your ability to represent the use of an inert electrode in a voltaic cell.
 Self-Check
Self-Check
SC 12. Complete “Practice” questions 1–9 on page 626 of the textbook.
1.7. Page 5
Module 4—Batteries and Balance
 Reflect and Connect
Reflect and Connect
In this lesson you learned to construct a voltaic cell. You also learned about the scientific principles involved in the design and operation of these cells. Read “Did You Know?” on page 615 and “Consumer, Commercial, and Industrial Cells” on pages 615–618 of the textbook. Do the different kinds of cells described in these readings have similar components to the voltaic cells you investigated in this lesson?
RC 1. Prepare a chart summarizing the similarities and differences you identified.
Save your chart in your course folder.
 Module 4: Lesson 1 Assignment
Module 4: Lesson 1 Assignment
There is no assignment for this lesson.
1.8. Page 6
Module 4—Batteries and Balance
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- How much do you rely on batteries in your everyday life?
- What are the components of a voltaic cell?
At the beginning of this lesson you conducted an audit of the different types of commercial cells used in your house. Did your reliance on commercial cells surprise you or did you just take for granted that battery use is part of modern-day life? How were you impacted when you calculated the mass of chemical waste generated by spent commercial cells disposed of by your class or by your community?
In this lesson you learned about the parts of a voltaic cell and how these parts work together to convert chemical energy into electrical energy. You learned how to use the “Table of Selected Standard Electrode Potentials” (also used in Module 3) to predict the strongest oxidizing and reducing agents in the system. You used the information from this table to write the half-reactions that occur while the cell is functioning. You also completed a virtual investigation in which you explored the connections between half-cells and the observable changes that occur when a voltaic cell operates.
1.9. Lesson 2
Module 4—Batteries and Balance
Lesson 2—Design of Commercial Cells
 Get Focused
Get Focused

Wow! There are a lot of different kinds of commercial electric cells available. Some cells, like a 9-volt cell, are unique because of their high voltage. But most of the cells you purchase have a voltage of 1.5 V. In addition to voltage, there are cells made with alkaline, rechargable, lithium ion, and metal hydride components.
In this lesson, you will investigate the construction of different types of commercial electric cells and other kinds of cells used commercially. As you proceed through this lesson, you will further consider the environmental impact of your personal use of commercial electric cells. You will collect information about how substances from discarded cells might enter the environment, and you will also collect information about mechanisms that can be used to reduce the quantity of material from these cells that goes into landfills.
Consider the following question as you complete Lesson 2:
- How do you determine the cell potential for a voltaic cell?
 Module 4: Lesson 2 Assignment
Module 4: Lesson 2 Assignment
In the Lesson 2 Assignment you will complete the investigation “Building Voltaic Cells.” Download a copy of the Module 4: Lesson 2 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.10. Page 2
Module 4—Batteries and Balance
 Explore
Explore
 Read
Read

When you look at different commercial electric cells you may notice that the cells come in many different shapes. While this can mean they have different voltages, in many cases (including in the picture shown) the cells have the same voltage. How is it possible to have cells with different shapes and the same voltage?
In Lesson 1 you constructed a zinc-copper voltaic cell, and you observed that it had a voltage of 1.10 V. You also analyzed the voltage of a copper-silver voltaic cell. Would you expect the copper-silver cell to have the same voltage as the zinc-copper cell?
As you may suspect, the choice of chemicals in your cell has a large impact on the cell's voltage. Do you think there might be a relationship between the voltage produced by a cell and the position of the reactants in the table of half-reactions you have been using throughout Unit B?
Read pages 627–630 in the textbook to learn more about voltages—standard cell potentials—produced by cells and the standard reduction potentials listed on the “Table of Selected Standard Electrode Potentials.” Then work through “Communication example 2” and “Communication example 3” on page 631 of the textbook.
 Self-Check
Self-Check
SC 1. Calculate the standard cell potential of a zinc-copper voltaic cell like the one you analyzed in Lesson 1.
SC 2. Calculate the standard cell potential of a copper-silver voltaic cell.
SC 3. Comment on the following observation: “A higher cell potential is observed with reactants that are greatly separated on the table of half-reactions.”
SC 4. Suggest a reason why the three types of consumer cells shown in the picture above have the same voltage, or cell potential.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
|
Half-Reaction |
Reduction |
Cu2+(aq) + 2 e– → Cu(s) |
Oxidation |
Zn(s) → Zn2+(aq) + 2 e– |
Eºcell = +0.34 V – (–0.76 V)
= +1.10 V
SC 2.
Half-Reaction |
|
Reduction |
Ag+(aq) + 1 e– → Ag(s) |
Oxidation |
Cu(s) → Cu2+(aq) + 2 e– |
Eºcell = +0.80 V – (+0.34 V)
= + 0.46 V
SC 3. The separation between the copper and zinc half-reactions is greater than the separation between the copper and silver half-reactions. This is consistent with the differences in cell potentials observed. Therefore, the observation is supported by the examples analyzed.
SC 4. The chemicals used in the three types of cells is the same, therefore the cell potentials should be identical.
 Read
Read
Sometimes the measured cell potential is not the same as the predicted standard cell potential. Read page 632 in the textbook to discover reasons for discrepancies you may encounter.
 Self-Check
Self-Check
SC 5. Complete “Practice” questions 12–16 on page 633 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 5.
Practice 12.
a. Pb(s) | Pb2+(aq) Cu2+(aq) | Cu(s)
The cathode is Cu(s); the anode is Pb(s).
![]()
b. Zn(s) | Zn2+(aq) Ni2+(aq) | Ni(s)
The cathode is Ni(s); the anode is Zn(s).
![]()
c. Pt(s) | H2(g), H+(aq) Fe3+(aq), Fe2+(aq) | Pt(s)
The cathode and anode are both Pt(s).
![]()
Note that H2 can reduce Fe3+ to Fe2+ but can’t reduce it all the way to Fe.
Practice 13.
Eºcell = Eºr(cathode) – Eºr(anode)
Eºr(anode) = Eºr(cathode) – Eºcell
![]()
The reduction potential of indium(III) ion is –0.34 V.
Practice 14.
For the copper half-cell, Eºr would become +3.38 V.
For the zinc half-cell, Eºr would become +2.28 V.
In the shift to this new reference point, all the reduction potentials must increase by the same amount (3.04 V) so that the differences between individual values remain constant.
Practice 15.
Differences could be caused by non-standard conditions of temperature, pressure, or concentration. Other possible factors include purity of substances, oxide coating on electrodes, and variations in the salt bridge or porous boundary.
Practice 16.
The cell “goes dead” because the reaction Zn(s) + Fe2+(aq) → Zn2+(aq) + Fe(s) has proceeded to the point of equilibrium. At this point there is a high concentration of Zn2+(aq) and a low concentration of Fe2+(aq).
Because you have not yet studied Module 7 on equilibrium, your answers may not be as complete as those provided here.
1.11. Page 3
Module 4—Batteries and Balance
 Read
Read

Chances are that the most-used type of commercial electric cell you found in the "battery" audit you conducted in Lesson 1 was a type of dry cell. In the Reflect and Connect section of Lesson 1 you read about “dry cells,” cells in which the quantity of water within the cell has been reduced, but the properties of the electrolytes have been maintained.
A modification of the dry cell is the alkaline dry cell. The modification did not affect the measured cell potential, but it did improve the performance of the cell. Go to “Table 2” on page 616 of the textbook and read the information comparing the dry cell to the alkaline dry cell. Can you classify the “Characteristics and uses” for the alkaline dry cell listed in the table according to perspective?
Alkaline cells use KOH as an electrolyte, which forms a basic, or alkaline, solution. Recall that as the concentration of OH- increases in a solution, the concentration of H+ is reduced. Basic electrolytes, therefore, reduce the possibility that H+ ions will act as an oxidizing agent in the battery. In other words, alkaline batteries tend to last longer before “going dead.”
Alkaline cells typically produce a potential that is comparable to a zinc-carbon cell (approximately 1.5 V). However, the electrolyte in a zinc-carbon cell is slightly acidic. This makes the zinc-carbon cell somewhat inefficient because both H+ ions and the intended oxidizing agent are reduced. Because of the low concentration of H+ ions and the undesirable reaction they promote, alkaline cells can deliver significantly higher currents. This combination of higher current and longer life is a very desirable characteristic for batteries that run devices like CD players, calculators, and smoke alarms.
Consumer cells are most often evaluated in terms of their durability and convenience. When it comes to batteries, social perspectives, which demand that portable communication devices like cellular telephones stay connected, have a much higher priority than, say, environmental perspectives, which consider the consequences of using all these batteries.
 Reflect on the Big Picture
Reflect on the Big Picture

![]() Retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will record your response to Reflect on the Big Picture in Part 1 of the Assignment document.
Retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will record your response to Reflect on the Big Picture in Part 1 of the Assignment document.
Look at the picture shown. Have you ever seen a cell in this condition? Sometimes an alkaline dry cell may leak contents as a result of a faulty seal in its construction. How should you deal with a leaking cell of this type? Use a variety of resources (e.g., textbook, Materials Safety Data Sheets, Internet) to research how a leaking alkaline dry cell should be safely removed from an electronic device and disposed of. Prepare disposal instructions that you could provide to a family member.
Save any information about concerns or procedures for disposal of waste from alkaline dry cells or other cells in your course folder. You may refer to this information later in this lesson as you consider the environmental impact of using these cells.
 Module 4: Lesson 2 Assignment
Module 4: Lesson 2 Assignment
If you have not already done so, retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete Part 1. You will receive instructions later in this lesson on when to complete the rest of the Assignment and when to submit your work to your teacher.
1.12. Page 4
Module 4—Batteries and Balance
 Module 4: Lesson 2 Lab—Building Voltaic Cells
Module 4: Lesson 2 Lab—Building Voltaic Cells
In this module you have learned a great deal about the construction of voltaic and other types of electrochemical cells. In this investigation you will construct voltaic cells, and you will use the techniques you have learned to test predictions that can be made about voltaic cells.
![]() Retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete the Part 2 Pre-Lab question now. You will complete the remainder of Part 2 after viewing the virtual investigation.
Retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete the Part 2 Pre-Lab question now. You will complete the remainder of Part 2 after viewing the virtual investigation.
![]() View the virtual investigation “Building Voltaic Cells.” Remember to record your data and observations as you view the presentation.
View the virtual investigation “Building Voltaic Cells.” Remember to record your data and observations as you view the presentation.
 Module 4: Lesson 2 Assignment
Module 4: Lesson 2 Assignment
If you have not already done so, retrieve your copy of the Module 4: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete all of Part 2, and save your work in your course folder. You will receive instructions later in this lesson on when to submit your work to your teacher.
1.13. Page 5
Module 4—Batteries and Balance
 Read
Read
Common Types of Commercial Cells

The warning on this battery reads “Caution. This battery is not rechargeable. May explode or leak if charged or if +- terminals are connected in reverse.”
Rechargeable cells are also popular in many applications including cellular telephones, video cameras, and personal music players.
One type of rechargeable cell is the nickel-cadmium cell. The advantage of nickel-cadmium batteries is that they have a relatively low internal resistance and can therefore supply high surges of current when necessary. They are also relatively easy to recharge. The main disadvantage of nickel-cadmium cells is that they typically have a lower cell potential—1.2 V as compared to alkaline dry cells, which have a measured potential of 1.5 V.

© Jupiterimages Corporation
Alkaline cells and other cells using zinc and manganese oxide as reactants are not rechargeable; some cells even come with warnings to prevent users from trying to recharge them. Later in this module you will learn more about the process of recharging cells and about why this process cannot be accomplished with all consumer cells.
Lithium ion cells are popular in small, portable devices like cellular telephones and digital cameras, which require short bursts of significant amounts of electrical energy. Lithium ion cells are typically composed of a lithium electrode, a graphite electrode (a form of carbon), and one of several possible electrolytes.
The most common wet cells in use today are lead-acid batteries used in automobiles. Lead-acid batteries are useful for automobiles because they are relatively cheap and easily recharged. The disadvantage of a lead-acid battery relates to its chemical contents—lead and concentrated sulfuric acid. Both substances can cause harm. Considerable caution needs to be exercised when this type of battery is transported, handled, and disposed of.
1.14. Page 6
Module 4—Batteries and Balance
 Reflect on the Big Picture
Reflect on the Big Picture

In Lesson 1 you completed an audit of the cells used in your home. In this lesson you investigated common types of consumer cells.
RBP 1. Retrieve your “battery” audit and classify the cells you counted into the following categories: rechargeable and non-rechargeable.
Many cells and batteries are discarded improperly. In this lesson you learned that some of the components of various types of cells can be toxic or may cause harm to the environment.
RBP 2. Research the following types of commonly used cells and batteries, and identify concerns about their disposal:
- alkaline dry cell (zinc-manganese oxide)
- nickel-cadmium
- nickel-metal hydride
- lithium ion
- lead-acid
Prepare a poster, information pamphlet, podcast, or other form of communication to promote safer practices for the disposal of used cells and batteries in your local area.
 Discuss
Discuss
Place a copy of your poster, pamphlet, or presentation in the discussion area for your class. Review the presentations of other students in your class, and provide constructive feedback to your classmates in the following areas:
- Is the scientific information correct?
- Does the presentation address concerns about the disposal of different types of spent cells?
- Are effective communication strategies used? How might the presentation of information be improved?
Review the feedback you receive on your own presentation, and revise your presentation. Submit your final presentation to your teacher.
 Module 4: Lesson 2 Assignment
Module 4: Lesson 2 Assignment
Retrieve your copy of the Module 4: Lesson 2 Assignment, and complete Part 3. Submit your completed assignment to your teacher.
1.15. Page 7
Module 4—Batteries and Balance
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following question:
- How do you determine the cell potential for a voltaic cell?
You learned how to determine cell potential for a voltaic cell using the electrical potentials that correspond with the half-reactions listed in the “Table of Selected Standard Electrode Potentials.” You completed an investigation to verify the predictions made for voltaic cells you constructed. You also investigated different types of consumer cells. You looked at the purpose of each type of cell and at concerns about their chemical components and proper disposal.
1.16. Lesson 3
Module 4—Batteries and Balance
Lesson 3—Electrolytic Cells
 Get Focused
Get Focused

Many of the devices you use daily rely on rechargeable electric cells. In this module you have learned about the ability of electric cells to convert chemical energy into electrical energy. What changes occur when the cell from your MP3 player or digital camera is “recharged”?
In this lesson you will learn about rechargeable cells. You will learn how a recharging cell is an example of a different type of electrochemical cell, and you will learn why some cells can be recharged while others cannot be recharged.
Consider the following questions as you complete Lesson 3:
- What is an electrolytic cell?
- What chemical and energy conversions occur within an electrolytic cell?
 Module 4: Lesson 3 Assignment
Module 4: Lesson 3 Assignment
In the Lesson 3 Assignment you will design and test an electrolytic cell. Download a copy of the Module 4: Lesson 3 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.17. Page 2
Module 4—Batteries and Balance
 Explore
Explore
 Read
Read
In previous science courses you may have seen how applying electrical energy to a solution caused chemical change. Electrolysis, the process of applying an electrical current to a system, is used to cause chemical reactions. In Lesson 2 you learned that electric cells, voltaic cells for example, involve a spontaneous electrochemical change. What kind of change occurs in an electrolytic cell? Read pages 639–640 in the textbook to learn about the scientific principles behind the operation of an electrolytic cell.
You may wish to save a copy of “Table 1” from page 640 in your course folder as a reminder of the similarities and differences between the two types of electrochemical cells: electric and electrolytic.
To get a clear understanding of the differences between the behaviours of electric and electrolytic cells, read “Secondary Cells: Electric and Electrolytic” on page 640 of the textbook. Does it make sense that a rechargable cell can be described as both an electric and an electrolytic cell, but that the cell can't be both at the same time?
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–4 on page 640 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1. At the cathode of an electrolytic cell, the oxidizing agent gains electrons and is reduced. At the anode of the cell, the reducing agent loses electrons and is oxidized.
Practice 2. In a voltaic cell, the cathode and anode are designated positive and negative respectively. In an electrolytic cell, the designations are the opposite.
Practice 3. In an electrolytic cell, electrons move through the external circuit from anode to cathode. Cations migrate toward the cathode, and anions migrate toward the anode.
Practice 4. The pumping of water into the tower is similar to the recharging of a secondary cell because energy is applied to drive a change (by applying a current to force a chemical reaction to occur in the cell). When water flows from the tower, it does so due to gravity. This spontaneous process is similar to the discharging of the secondary cell in which the chemical energy is converted into electrical energy.
 Try This
Try This
Electrolysis of Potassium Iodide
In the next activity you will watch a video showing the electrolysis of a solution. The system you will observe is an aqueous potassium iodide solution that will be placed in a U-tube. A carbon electrode will be placed in each end of the tube. The carbon electrodes will be connected to a source of electricity.
Complete the following questions about the system before you view the video. The questions will prepare you to anticipate the events and changes you will observe in the video. As you complete the questions, recall the techniques that you used to predict redox reactions and that you applied in your study of voltaic cells. Remember to carefully scan the states of substances in half-reactions, as they may help you identify observable changes that will occur as the cell operates.
TR 1. What are the strongest oxidizing and reducing agents in this system?
TR 2. What are the half-reactions for the strongest oxidizing and reducing agents in this system?
TR 3. Describe one observable change you would expect to see at each of the electrodes, and describe a diagnostic test you could perform to confirm the result.
TR 4. Calculate the Eºcell for this system. What does this value represent in terms of operating the cell?
 Watch and Listen
Watch and Listen
View the video “
You may wish to read pages 641–642 in the textbook. This reading provides an in-depth explanation of the events observed in the video and will also confirm your answers to questions TR 1–TR 4.
 Read
Read
Read “Summary” on page 642 of the textbook. Work through “Communication example 1” and “Communication example 2” on pages 643 and 644.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 5 and 6 on page 644 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 5.
a. cathode: Ni2+(aq) + 2e– → Ni(s)
anode: 2 I–(aq) → I2(aq) + 2e–
net cell: Ni2+(aq) + 2 I–(aq) → Ni(s) + I2(aq)
A minimum voltage of +0.80 V is required.
b. cathode: (2 H2O(l) + 2e– → H2(g) + 2 OH–(aq)) × 2
anode: 4 OH–(aq) → O2(g) + 2 H2O(l) + 4e–
net cell: 2 H2O(l) → 2 H2(g) + O2(g)
![]()
A minimum voltage of +1.23 V is required.
Practice 6.
a. ![]()
The minimum voltage required is +1.48 V. Note that the oxidizing agent is Cr3+ and the reducing agent is Br-.
b. ![]()
The minimum voltage required is +0.89 V. Note that the oxidizing agent is Cu2+ and the reducing agent is H2O.
 Self-Check
Self-Check
In previous science courses you may have seen the electrolysis of water that makes hydrogen and oxygen gases. In Module 3 you learned about disproportionation reactions, a type of electrochemical reaction in which the same species acts as the oxidizing and the reducing agent. The electrolysis of water is another example of a disproportionation reaction.
SC 3. What are the oxidation and reduction half-reactions involved in the electrolysis of water?
SC 4. What technical consideration is necessary to make the electrolysis of water possible?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
reduction (cathode): 2 H2O(l) + 2 e– → H2(g) + 2 OH–(aq)
oxidation (anode): 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e–
SC 4. Pure water does not conduct an electric current; therefore, a flow of charge would not be possible within an electrolytic cell containing pure water. Often the water has a small quantity of sodium sulfate added to it prior to electrolysis. The sodium ions and the sulfate ions in the system are spectator ions, but they are essential to allowing the cell to complete the flow of charge necessary for the transfer of electrons to occur.
 Self-Check
Self-Check

In a laboratory, the device used to collect the gases produced by an electrolysis is called a Hoffman’s apparatus. The solution within the apparatus is electrolyzed, and the gases are collected in a chamber above each of the electrodes.
SC 5. Predict the products of the electrolysis of an aqueous sodium chloride solution using a Hoffman’s apparatus.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answer varies significantly from the answer provided here.
SC 5. The strongest oxidizing and reducing agent in this system is water; therefore, the expected products are as follows:
- hydrogen gas and hydroxide ions (cathode; basic pH)
- oxygen gas and hydrogen ions (anode; acidic pH)
 Watch and Listen
Watch and Listen
View the video “
 Read
Read
Read “Evaluation of Predicted Reactions—The Chloride Anomaly” on page 645 of the textbook to learn about the unique result obtained from the electrolysis of aqueous chloride solutions.
1.18. Page 3
Module 4—Batteries and Balance
 Read
Read
What is the connection between electrolysis and the “recharging” that occurs within a rechargeable commercial cell? A rechargeable commercial cell does not allow new materials to enter or leave the cell. Therefore, the application of electrical energy used in recharging the cell must create a chemical change. The chemical change recreates the reactants of the spontaneous reaction that produces the flow of electrons. The spontaneous reaction that produces the flow of electrons is referred to as the discharge of the cell.
The reactions for the discharge of the cell and its recharging are reversible. The products of one process become the reactants for the next process. The process of discharging and recharging can be repeated many times, provided an energy source is available for the non-spontaneous recharging process, and that the half-reactions in the discharging process are reversible and recreate the reactants.
 Reflect on the Big Picture
Reflect on the Big Picture
Use the information in the Read section above to complete the following questions. Use complete descriptions. Where possible, make reference to the information provided to support your answer.
RBP 1. The discharge of a Ni-Cd rechargeable cell is described by the following half-reactions:
2 NiO(OH)(s) + 2 H2O(l) + 2 e– → 2 Ni(OH)2(s) + 2 OH–(aq) Eºnet = +0.49 V
Cd(s) + 2 OH–(aq) → Cd(OH)2(s) + 2 e– Eºnet = –0.81 V
Arrange the half-reactions to determine the net reaction and the net cell potential for the discharge of a Ni-Cd cell. What are the products of the recharging process, and what is the minimum voltage that must be applied to a recharging Ni-Cd cell?

The warning label reads “This battery is not rechargeable. May explode or leak if charged or it +- terminals are connected in reverse.”
RBP 2. The half-reactions for a common type of dry cell are shown below:
cathode: MnO2(s) + H2O(l) + 2 e– → Mn2O3(s) + 2 OH–(aq)
anode: Zn(s) → Zn2+(aq) + 2 e–
The cell shown in the photograph has a prominent warning label. Provide reasons for the warning shown in the picture, and explain why it is not physically possible to recharge this type of cell.
Save your answers in your course folder. Submit a copy of your answers to you teacher for feedback.
 Module 4: Lesson 3 Assignment
Module 4: Lesson 3 Assignment
Retrieve your copy of the Module 4: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete all questions. Save your completed Assignment in your course folder and submit a copy to your teacher.
1.19. Page 4
Module 4—Batteries and Balance
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- What is an electrolytic cell?
- What chemical and energy conversions occur within an electrolytic cell?
You found that although electrolytic and electric cells share many of the same characteristics, they have important differences. The table shown, from page 640 of the textbook, summarizes the similarities and differences between the voltaic cell (a type of electric cell) and the electrolytic cell.
Table 1: Comparing Electrochemical Cells: Voltaic [(a type of electric cell)] and Electrolytic
| Voltaic [(a type of electric)] cell | Electrolytic cell | |
|---|---|---|
| spontaneity | spontaneous reaction | nonspontaneous reaction |
| standard cell potential Eºcell |
positive | negative |
| cathode |
|
|
| anode |
|
|
| direction of electron movement | anode → cathode | anode → cathode |
| direction of ion movement |
|
|
In an electrolytic cell, electrical energy is applied to create chemical change. You learned that rechargeable cells, sometimes called secondary cells, are one type of electrolytic cell. Other electrolytic cells are used to produce gases like oxygen, hydrogen, and chlorine. In the next lesson you will learn more about how electrolytic cells are used to produce and refine metals.
1.20. Lesson 4
Module 4—Batteries and Balance
Lesson 4—Commercial Applications of Electrolytic Cells
 Get Focused
Get Focused

In Lesson 3 you saw how an electrolytic cell can be used to produce pure elements such as the gases hydrogen, oxygen, and chlorine, and pure metals like copper. The electrolytic cell causes chemical change by forcing a redox reaction to occur.
In previous science courses you may have seen a demonstration of the reactivity of sodium, potassium, and lithium (group 1 metals) with water. Do you recall which metals reacted most vigorously—so much so that you could see a flame? How could such reactive metals ever be converted into their elemental forms? Is it possible that an electrolytic cell could be used for the production of all metals?
In Lesson 3 you observed the operation of laboratory-scale electrolytic cells. How is the electrolytic cell used to plate chromium onto automobile bumpers, as shown in the photograph, similar and different to the cells you build in a laboratory? In this lesson you will investigate society's use of electrolytic cells as a technology.
Consider the following question as you complete Lesson 4:
- What are some of the practical applications of electrolytic cells?
 Module 4: Lesson 4 Assignment
Module 4: Lesson 4 Assignment
There is no assignment for this lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.21. Page 2
Module 4—Batteries and Balance
 Explore
Explore
 Read
Read
The development of electric cells was essential to experimentation using electrolytic cells. To learn more about this relationship and how electrolysis can be used to produce elements, read pages 646–647 in the textbook. Stop reading when you get to the end of “Communication example 3” on page 647.
The reading introduces an important aspect of the electrolysis of weak oxidizing agents like the ions of group I metals. Because water is a stronger oxidizing agent than Na+(aq), K+(aq), and Li+(aq) the production of metals in group I can only be achieved in molten conditions. You will recall that ionic compounds also tend to have high melting temperatures, normally well above the boiling point of water. When molten, ionic compounds such as NaCl(l) act as an electrolyte because ions are free to move. As you have already learned in this module, the movement of ions is essential for the conservation of charge in both electric and electrolytic cells.
Read “Production of Aluminium” on pages 647–648 in the textbook to see how an electrolytic cell containing molten contents is designed and operated. Then read “The Chlor-Alkali Process” on page 648 to learn how chlorine is produced at an industrial scale in a facility such as Dow Chemical in Fort Saskatchewan, Alberta.
In Unit C of this course you will learn how chlorine produced by the chlor-alkalai process can be used in chemical reactions with hydrocarbons to make plastics. The partnering of industrial processes like the chlor-alkali process with the refining of petroleum is an important part of Alberta’s petrochemical industry.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 16–18 and 21 on page 649 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 16.
From an aqueous solution, trying to reduce a metal ion (which is a weaker oxidizing agent than water) is futile. When water is the strongest oxidizing agent present, it will be reduced and the metal ion will remain unchanged in the solution. Also, many ionic compounds have low solubility in water, which makes it impractical to electrolyze an aqueous solution to obtain a metal. The technological solution is to conduct the electrolysis in the absence of water; which is possible by melting the ionic compound.
Practice 17.
cathode: 2[Sc3+(l) + 3 e– → Sc(s)]
anode: 3[2 Cl–(l) → Cl2(g) + 2 e–]
net cell: 2 Sc3+(l) + 3 Cl–(l) → 2 Sc(s) + 3 Cl2(g)
Practice 18.
cathode: 2 H2O(l) + 2e– → H2(g) + 2 OH–(aq)
anode: 2 Cl–(aq) → Cl2(g) + 2e–
net cell: 2 H2O(l) + 2 Cl–(aq) → H2(g) + 2 OH–(aq) + Cl2(g)
Practice 21.
Recycling aluminium has many benefits. To recycle an aluminium can, it takes only 5% of the energy required to produce a new one. Because every method for producing electrical energy has negative consequences, this energy saving reduces the aluminium industry’s impact on the environment. Recycling aluminium also saves bauxite, the mineral from which aluminium is obtained. This preserves bauxite for future generations. Recycling also reduces the amount of waste going into landfills.
Read

Electrolytic cells can also be used to refine metals. Read “Refining of Metals” on pages 649–650 of the textbook.
Many people have been attracted to the glowing chrome on classic and customized automobiles. The process of placing chromium over the steel used to construct bumpers and other parts of a car’s body is called electroplating.
 Watch and Listen
Watch and Listen
View the virtual investigation “Electroplating Copper.” This investigation is a laboratory-scale example of electroplating.
TR 1. As you view the investigation, record qualitative and quantitative data observed.
Save a copy of the data in your course folder. You will use the data in Lesson 5 to complete an analysis of this investigation.
 Try This
Try This
The following questions are based on the virtual investigation you just completed, “Electroplating Copper.”
TR 2. Describe any changes that occurred at the carbon electrode.
TR 3. Describe any changes that occurred at the copper electrode.
TR 4. Identify the evidence that indicates a reduction half-reaction occurred during the operation of the electrolytic cell. Write the half-reaction that describes this reaction.
TR 5. Identify the evidence that indicates an oxidation half-reaction occurred during the operation of the electrolytic cell. Write the half-reaction that describes this reaction.
TR 6. Draw a diagram of the apparatus. Label the anode, cathode, electrolyte, and direction of electron flow provided by the power source.
 Read
Read
Read “Electroplating” on page 650 of the textbook.
 Self-Check
Self-Check
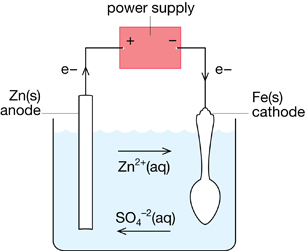
SC 2. Complete “Section 14.3” question 15 on page 651 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answer varies significantly from the answer provided here.
SC 2.
Section 14.3 15.
Electroplating Zinc onto an Iron Spoon
1.22. Page 3
Module 4—Batteries and Balance
 Reflect on the Big Picture
Reflect on the Big Picture
Society uses a lot of metal. That makes the industries that use electrolytic cell technology to produce elements and refine and plate metals extremely important. There are toxicity issues associated with some metals and substances used in electroplating. Careful treatment of chemical waste is essential if these industries are to demonstrate sustainability.
You may wish to investigate whether any businesses in your local area electroplate or recycle metals (recycling also often uses electroplating). How do these businesses deal with chemical waste? What provincial and federal laws apply to these businesses? How do these businesses address concerns about protecting the environment?
 Module 4: Lesson 4 Assignment
Module 4: Lesson 4 Assignment
There is no assignment for this lesson.
1.23. Page 4
Module 4—Batteries and Balance
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following question:
- What are some of the practical applications of electrolytic cells?
You learned that electrolytic cells are used to produce elements, refine metals, and electroplate. In the next lesson you will learn about quantitative relationships that need to be considered when operating an electrolytic cell. Can you think of what chemical entity in an electrolytic cell determines the quantity of product that can be produced? Go to Lesson 5 to learn the answer.
1.24. Lesson 5
Module 4—Batteries and Balance
Lesson 5—Quantitative Relationships in Cells

© Laurence Gough/shutterstock
 Get Focused
Get Focused
In Lesson 4 you learned that electrolytic cells can be used to produce measurable quantities of elements, including gases and electroplated metals. As you completed the virtual investigation “Electroplating Copper,” did you find yourself wondering about the changes to the mass of each electrode in the cell? In this lesson you will revisit the data you collected from the Lesson 4 virtual investigation, and you will research other electrolytic cells. You will investigate the stoichiometric relationships involved.
From your work in previous chemistry courses and in earlier modules of this course, you will recall that stoichiometry is a process that can be used to predict and explain the chemical and measurable quantities of matter involved in a process. Can you think of how stoichiometric principles could be applied to an electrochemical reaction occurring in an electric or electrolytic cell? Is it possible to have a quantitative relationship between electrons and the atoms involved?
Consider the following question as you complete Lesson 5:
- What are the important quantitative relationships within operating electric and electrolytic cells?
 Module 4: Lesson 5 Assignment
Module 4: Lesson 5 Assignment
Download a copy of the Module 4: Lesson 5 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.25. Page 2
Module 4—Batteries and Balance
 Explore
Explore
 Read
Read
Earlier in this module you were asked to list similarities and differences between electric and electrolytic cells. One important similarity is that both cell types need a transfer of electrons in order for any chemical change to occur. You tested this when you constructed a voltaic cell and observed the change in the voltmeter reading when the cell's circuit was broken by briefly removing various components. In an electrolytic cell, you observed that a chemical change only occurs when the power source is turned on and a voltage greater than the minimum cell potential is applied. Is it possible to identify what chemical entity controls the change observed in other species? What do these observations tell you about electrochemical cells in terms of quantitative relationships?
You probably haven't considered electrons as quantifiable. However, when you wrote half-reactions, you used coefficients to indicate the proportions of electrons involved in an oxidation or reduction. Read the text and work through the “Sample problems” and “Communication examples” on pages 652–654 in the textbook to learn more about the methods used to quantify electrons.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–4 on page 653 of the textbook and “Practice” questions 5–7 on page 654 of the textbook.
 Read
Read
In the previous section you learned how to use measurements of amperage and time to calculate moles of electrons. As you will recall, a balanced chemical reaction, including a half-reaction, provides information about the proportions of substances in a chemical process. With knowledge of the quantity of electrons, you will be able to complete the same kinds of stoichiometric calculations performed elsewhere in this course to determine the quantities of other species in the half-reaction.
Read pages 655–656 in the textbook. Work through “Sample problem 14.4” and “Communication example 3” to learn more about performing these calculations.
 Self-Check
Self-Check
SC 2. Complete “Section 14.4” questions 1–3, 9, 11, and 12 on page 657 of the textbook.
 Module 4: Lesson 5 Assignment
Module 4: Lesson 5 Assignment
Retrieve your copy of the Module 4: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save a copy of your completed Assignment in your course folder. You will receive instructions later in this lesson on when to submit your Assignment to your teacher.
 Try This
Try This
Earlier in this module you investigated voltaic cells. Could the calculations involving amperage, time, and moles of electrons be applied to predicting the changes involved in the operation of a voltaic cell?
TR 1. Demonstrate the application of stoichiometry to predicting a quantitative relationship in a voltaic cell of your choice. The cell can be a cell described in the textbook or in other resources, or a cell with reactants of your choice. Your demonstration can be presented in a format similar to the “Sample problems” or “Communication examples” in the textbook.
Save a copy of your answer in your course folder. Submit a copy of your answer to your teacher for feedback.
1.26. Page 3
Module 4—Batteries and Balance
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you learned how to use quantitative information related to the operation of an electrochemical cell to predict the amount of species participating in the reaction. Throughout this module you have considered the environmental impact of electrochemical processes. Like other chemical processes, the properties and quantity of chemical waste are important in determining appropriate treatment methods.
Future research in the area of environmental chemistry or in other scientific areas may change opinions regarding the harm that some forms of chemical waste may cause. For example, alkaline dry cells collected at Eco Station facilities in Edmonton are currently placed in a special landfill; depending on future developments in technology for recycling, these alkaline dry cells may be unearthed and processed. It is very possible that the processing of waste from alkaline dry cells will involve further electrochemical reactions, possibly even electrolysis. Therefore, a knowledge of quantitative relationships in electrochemical processes is certain to be an important part of any efforts to recycle this type of waste.
 Module 4: Lesson 5 Assignment
Module 4: Lesson 5 Assignment
Submit your completed Module 4: Lesson 5 Assignment to your teacher.
1.27. Page 4
Module 4—Batteries and Balance
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following question:
- What are the important quantitative relationships within operating electric and electrolytic cells?
This lesson extended your application of the stoichiometric method to include amperage and time. You used the stoichiometric method to determine the chemical quantity of electrons participating in a reaction. Since electrons determine the chemical changes in these cells, they can be treated as the limiting reactant in many electrochemical systems. As such, electrons can be used to calculate the quantities of products or reactants in the system that participates in the process.
1.28. Module Summary/Assessment
Module 4—Batteries and Balance
 Module Summary
Module Summary
In this module you investigated technological applications of the scientific principles associated with reduction–oxidation reactions.
You considered the following module question:
- How are principles of oxidation and reduction applied to electrochemical cells?
You used your understanding of electron transfer reactions and skills and techniques to predict, explain, and interpret chemical changes in order to investigate electric (e.g., voltaic) and electrolytic cells.
As you found with the chemical systems you investigated in Module 3, the chemical behaviour of both electric and electrolytic cells can be explained using half-reactions, net ionic equations, and cell potentials. You paid careful attention to the products and reactants in the equations you wrote. This allowed you to make observations that confirmed your predictions for both types of electrochemical cells.
You also learned to apply your knowledge of stoichiometry to reduction-oxidation reactions occurring in these cells. You extended your understanding of reaction chemical entities by using information about amperage and time to calculate moles of electrons participating in a chemical process. In the case of an electrolytic cell, you regarded electrons as the limiting reagent in the cell’s operation.
Concept Map or Graphic Organizer
As you worked through Module 4, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 4 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 4 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
![]](https://moodlehub.ca/pluginfile.php/3027/mod_book/chapter/6625/chemistry_30/ub/icons/assignment.gif) Module Assessment
Module Assessment

© 2008 Jupiterimages Corporation
When you were in elementary school, you might have constructed an electrochemical cell using a potato or a lemon. You have come a long way in understanding electrochemical cells. In this Assessment you will demonstrate your understanding of the scientific principles and technical knowledge involved in the design and operation of electrochemical cells.
With an electrochemical cell made from a potato or a lemon in mind, investigate ONE of the following research questions:
- Do all cells of this kind produce the same voltage?
- Do all cells of this kind operate for the same length of time?
- Can different voltages be recorded from cells constructed using the same fruit or vegetable?
- Is it possible to make these kinds of cells using a wide range of fruits and vegetables?
Read through the parts of conducting an experiment described in the table provided. Formally document your work in two of these steps, and submit your documentation to your teacher for assessment. Document your work in the format that best allows you to provide accurate, detailed, and complete descriptions of your work; e.g., video, audio, text, or a combination of formats.
Part |
Criteria |
Planning an Experiment |
|
Performing an Experiment |
|
Analysis of Experimental Work/Design |
|