Module 7
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 7 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 11:18 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 7
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Lesson 2
- 1.8. Page 2
- 1.9. Page 3
- 1.10. Page 4
- 1.11. Page 5
- 1.12. Lesson 3
- 1.13. Page 2
- 1.14. Page 3
- 1.15. Page 4
- 1.16. Lesson 4
- 1.17. Page 2
- 1.18. Page 3
- 1.19. Page 4
- 1.20. Page 5
- 1.21. Lesson 5
- 1.22. Page 2
- 1.23. Page 3
- 1.24. Page 4
- 1.25. Page 5
- 1.26. Lesson 6
- 1.27. Page 2
- 1.28. Page 3
- 1.29. Page 4
- 1.30. Page 5
- 1.31. Module Summary/Assessment
- 1.32. Module Glossary
1. Module 7
Module 7—Principles of Chemical Equilibrium
Module Introduction

© 2008 Jupiterimages Corporation
In Module 7 you will examine chemical equilibrium. Chemical equilibrium is something you may not have thought much about, but it is present and it affects many of the chemical systems you have investigated in this course. For example, in the last module you prepared esters. When you cooled the esters on ice, you were manipulating an equilibrium so that you would be better able to collect and observe the ester you had synthesized.
Other examples of chemical equilibrium are found in biological systems, chemical interactions, and industrial applications. In this module you will investigate many systems and how they demonstrate the principles of systems at equilibrium. You will learn how equilibrium can be classified, studied, and manipulated. You will learn how to predict changes to equilibrium when a system that appears to remain constant is stressed, and you will learn the scientific principles involved when systems are stressed.
In Module 7 you will investigate the following questions:
- What is happening in a system at equilibrium?
- How do scientists predict shifts in the equilibrium of a system?
As you approach the end of the module, you will come to understand many aspects of equilibrium and how systems in equilibrium behave. You will be asked to pay special attention to specific properties of each of the systems you investigate.
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 7, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit D Concept Organizer. In the Module 7 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 7—Principles of Chemical Equilibrium
 Big Picture
Big Picture

© 2008 Jupiterimages Corporation
In the Module Introduction you read that there are examples of equilibrium all around you. You are probably questioning whether this is true. In this module you will be introduced to a number of chemical systems in which equilibrium is critical to each system’s function.
Studying equilibrium is not only a matter of knowing the system; studying equilibrium also involves knowing the changes that can occur within the system that help to describe the equilibrium. In your study you will learn about forward and reverse reactions, conditions, and stresses; all of which define a chemical equilibrium. You will do more than study the basic principles of a system at equilibrium; you will learn how manipulating conditions can allow you to understand a chemical equilibrium.
Keep detailed notes. You will use your notes to complete the Module Assessment. In Reflect on the Big Picture activities at the end of each lesson, you will be prompted to construct and add to a table that lists the different systems you investigate. As you update your table you will revisit the concepts you learned in previous lessons, and you will apply that knowledge to new systems. This is a great way to keep using the knowledge you gain in each lesson. You will also be able to use this table to prepare for assessments.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete, and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 7 Assessment you will analyze an equilibrium system, and you will design an experiment to further investigate the factors that influence its equilibrium.
More information is provided in the Module Assessment. You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 7—Principles of Chemical Equilibrium
In This Module
Lesson 1—Introduction to Equilibrium
In Lesson 1 you will learn about the two types of equilibrium and how to differentiate between them. You will learn to identify the characteristics of chemical systems that are at equilibrium.
You will investigate the following lesson questions:
- What is equilibrium?
- What is happening in a system that is in equilibrium?
- How can you determine whether a system is open or closed?
Lesson 2—Dynamic Equilibrium
In Lesson 2 you will learn about the three types of equilibrium for chemical systems, and you will learn to identify forward and reverse processes in each.
You will investigate the following lesson questions:
- What is a reverse reaction and why is it important in an equilibrium system?
- What changes occur during dynamic equilibrium?
Lesson 3—Position of an Equilibrium
In Lesson 3 you will learn how an equilibrium can be established and what the relative proportions of products to reactants can be in a system at equilibrium.
You will investigate the following lesson question:
- Are all equilibrium states similar?
Lesson 4—Quantitative Perspectives of Equilibrium
In Lesson 4 you will learn to apply your skills in performing stoichiometric calculations to analyze chemical systems in equilibrium.
You will investigate the following lesson question:
- What procedures are used to calculate the chemical quantities for all substances in a chemical system?
Lesson 5—The Equilibrium Constant
In Lesson 5 you will learn about the equilibrium constant and the equilibrium law. The equilibrium law is an expression that relates the concentrations of the products to the reactants in a chemical system. It is used to calculate an equilibrium constant. You will come to understand how the value for the equilibrium constant is used to communicate information about the position of an equilibrium.
You will investigate the following lesson questions:
- What is the equilibrium constant?
- What information about an equilibrium is provided in an equilibrium constant?
Lesson 6—Le Châtelier’s Principle
In Lesson 6 you will learn how chemical systems at equilibrium react to the application of a stress. The stress can be a change to the concentration of one of the species in the system, a change in the system’s temperature, or a change in the volume (pressure) of the system. You will learn how to predict shifts in equilibrium and to identify the purpose of predicting and manipulating equilibrium by causing shifts.
You will investigate the following lesson question:
- What is Le Châtelier’s principle and how is it used to predict changes in chemical equilibrium systems?
1.3. Lesson 1
Module 7—Principles of Chemical Equilibrium
Lesson 1—Introduction to Equilibrium
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
It’s one of the last long weekends of the summer and you are spending the weekend beside a lake. Unfortunately, a rain shower is approaching, and it looks like you will have to take a break from your lakeside activities. Fortunately, there is plenty to do inside the cabin with your friends. Maybe you will get a seat at the card game that started a few hours ago.
It might not seem immediately obvious, but systems at equilibrium are all around you. Can you identify any systems at equilibrium in the situation described in the paragraph above?
To identify a system at equilibrium, you have to know the characteristics that define equilibrium. In this lesson you will begin to investigate equilibrium in chemical systems by learning about the different types of equilibrium that can exist. You will also learn the conditions that define a system at equilibrium.
Consider the following questions as you complete Lesson 1:
- What is equilibrium?
- What is happening in a system that is in equilibrium?
- How can you determine whether a system is open or closed?
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
There is no assignment for this lesson. However, you will begin a table to track the many chemical systems you will investigate and what you learn about their equilibrium. You will add to your table throughout Module 7, and you will use your table to complete the Module Assessment. You will be prompted to begin your table in the Reflect on the Big Picture activity towards the end of this lesson.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore

© 2008 Jupiterimages Corporation
What defines an equilibrium? You may be surprised to learn that an equilibrium is defined as a system that has constant properties and appears not to change. In the lakeside example from Get Focused, the water level of the lake and the quantity of oxygen dissolved in the lake’s water are two examples of equilibria that exist. Can you think of other examples of systems around you that appear not to change and may be equilibrium systems?
Reconsider the example of the lakeside cabin. Either during a rainstorm or at night when all your friends have come inside and no one leaves or enters, an equilibrium has established in the cabin. The equilibium has established because the number of people in the cabin remains constant. Because no one can come or go, the system (the cabin and its occupants) is a closed system.
Equilibrium can appear in one of two ways: as a static equilibrium or as a dynamic equilibrium.
During the evening, the position of people inside the cabin could resemble a dynamic equilibrium. This would be true if the number of people playing cards inside the cabin, at any given time, were to remain the same. Over the evening, people could enter and leave the card game, but the number of people at the table at any given time would have to remain constant. As you can see from this example, in a dynamic equilibrium there is a change, but the change is balanced by two processes. In this example the balance is with the number of people entering and leaving the card game; for every person that enters, one person must leave so that the number of people in the game remains constant.
equilibrium: the state of a closed system in which the system does not appear to change
For a chemical system, physical properties, like colour, remain constant and concentrations of substances within the system do not change.
static equilibrium: the idea that forces on an object are opposing and equal
The object remains constant because no changes are happening internally.
dynamic equilibrium: the idea that there is a balance between two opposing processes (forward and reverse) occurring at the same rate
The balance between the forward and reverse processes maintains the system’s appearance of constant properties, in spite of change that has occurred internally.
closed system: a system that is separated from its surroundings by a definite boundary so that energy can enter and leave the system but matter cannot
macroscopic: can be observed with the unaided eye or other senses
microscopic: cannot be observed with the unaided eye or other senses
Now consider the lakeside cottage system more broadly. Consider the closed system to include the cabin, its immediate surroundings, and the number of people not only in the cabin, but in the immediate surroundings as well. In this broader system, no one comes or goes. As such, it is a static system. In the example of this broader system, it would be possible to have a change in microscopic properties without having a change in macroscopic properties.
You may also have noticed that these examples describe not only static and dynamic equilibrium, but they also describe two different equilibrium states. For example, the number of people expected to be in the cabin when the weather is sunny, is much different than it would be during a rainstorm or at night. Each of these situations is an equilibrium because of the observation of a constant number of people inside the cabin. However, these two equilibrium states are different. Can you think of a reason to account for the difference between the two equilibrium states?
All systems, including those at equilibrium, are influenced by external forces and conditions. Both rain and night are conditions that appear to encourage people to congregate inside the cabin. Under these conditions you can expect one equilibrium state. When the conditions change, so can the equilibrium.
open system: a system in which both matter and energy can enter and leave the system
Can you predict what might happen to the equilibrium situation of people in the cabin if you expect more friends to arrive throughout the day? As you might expect, adding more people means that your system is no longer closed. An open system is one in which matter can be exchanged with its surroundings. In an open system it is much more difficult to predict changes in equilibrium, since it is more difficult to isolate the matter you wish to study.
 Read
Read
Read pages 674–676 in the textbook to learn more about the different types of equilibrium and systems.
 Try This
Try This
Do All Reactions Go to Completion?
Purpose
In this investigation you will observe videos of two chemical systems, and you will write reaction equations to describe the chemical changes you observe.
Step 1: View “Reaction 1: HCl(aq) + Mg(s)” to observe the components of the system and the change that occurs. Write a balanced chemical equation to describe the changes observed. You may wish to view the video more than once to obtain all the information you need.
Step 2: Review the exploration “Shakin’ the Blues” on page 675 of the textbook. In this system the progress of the chemical reaction is monitored using the colour of the system. Methylene blue indicator in its oxidized form (which can be represented as MBox) appears blue. In its reduced form (which can be represented as MBred), methylene blue indicator appears colourless.
Step 3: View “Reaction 2: Shaking the Blues” to observe the components of the system and the change that occurs. Write a balanced chemical equation to describe the changes observed. As you watch the video, observe changes to the colour of the system. What do your observations suggest about the completeness of the reaction and your understanding of chemical equilibrium?
Analysis
TR 1. Which reaction(s) proceeded to completion?
TR 2. How did you describe whether a reaction proceeded to completion in the chemical equations you wrote? How did you describe a reaction that did not appear to be complete?
TR 3. Which system(s) demonstrated that an equilibrium had been established? Explain your reasoning.
Save your responses in your course folder, and submit a copy to your teacher.
1.5. Page 3
Module 7—Principles of Chemical Equilibrium
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you have examined some chemical systems, and you have learned about the criteria that define an equilibrium system. In the remaining Module 7 lessons you will investigate more chemical systems and other aspects of equilibrium.
Create a table to summarize what you have learned about the systems so far. You will add to this table throughout Module 7, and you will use your table for the Module Assessment.
Procedure
RBP 1. Create a table like the following:
System |
Open or Closed? |
Observation of Macroscopic Changes? |
Observation of Microscopic Changes? |
Cabin and Friends |
|
|
|
HCl(aq) + Mg(s) |
|
|
|
Methylene Blue, Glucose, Potassium Hydroxide, and Oxygen |
|
|
|
RBP 2. Fill in the table with the systems you investigated in this lesson and your observations for each system.
 Reflect and Connect
Reflect and Connect
RC 1. Take some time as you go through your day to note systems in your life that are examples of either static or dynamic equilibrium. Choose the best examples and describe why each one meets the criteria for a system at equilibrium. Summarize your examples on your table of equilibrium systems.
![]() Post your examples of systems at equilibirum to the discussion area for your class. Review the postings of at least two other students. Provide feedback to help the students improve the precision of their examples. Revise your examples using feedback you receive from other students. Save your revised examples in your course folder.
Post your examples of systems at equilibirum to the discussion area for your class. Review the postings of at least two other students. Provide feedback to help the students improve the precision of their examples. Revise your examples using feedback you receive from other students. Save your revised examples in your course folder.
 Module 7: Lesson 1 Assignment
Module 7: Lesson 1 Assignment
There is no assignment for this lesson.
1.6. Page 4
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- What is equilibrium?
- What is happening in a system that is in equilibrium?
- How can you determine whether a system is open or closed?
You learned that equilibrium is defined as a system having constant properties and appearing not to change. There are two main types of equilibrium: static equilibrium and dynamic equilibrium. In a dynamic equilibrium there is a balance between two opposing processes, the forward and reverse reactions, both occurring at the same rate. You also learned to differentiate between a closed system and an open system.
You applied your newly acquired knowledge to identify examples of equilibrium systems. As you work through Module 7 you will continue to examine these systems to describe their other attributes, and you will consider how these attributes are similar in all equilibrium systems.
Lesson Glossary
closed system: a system that is separated from its surroundings by a definite boundary so that energy can enter and leave the system but matter cannot
dynamic equilibrium: the idea that there is a balance between two opposing processes (forward and reverse) occurring at the same rate
The balance between the forward and reverse processes maintains the system’s appearance of constant properties, in spite of change that has occurred internally.
equilibrium: the state of a closed system in which the system does not appear to change
For a chemical system, physical properties, like colour, remain constant and concentrations of substances within the system do not change.
macroscopic: can be observed with the unaided eye or other senses
microscopic: cannot be observed with the unaided eye or other senses
open system: a system in which both matter and energy can enter and leave the system
static equilibrium: the idea that forces on an object are opposing and equal
The object remains constant because no changes are happening internally.
1.7. Lesson 2
Module 7—Principles of Chemical Equilibrium
Lesson 2—Dynamic Equilibrium
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
In Lesson 1 you observed the colour changes of methylene blue that resulted when the system the indicator was in was shaken or allowed to sit undisturbed. In your analysis of that system you learned that chemical reactions are reversible.
To some extent all chemical systems demonstrate reversibility. Therefore, studying the equilibrium of chemical systems involves recognizing the forward and reverse reactions for a system and their reaction rates. For example, if the evaporation of water into the atmosphere is considered the forward reaction for the water in the biosphere system, then precipitation as rain, snow, or fog would be the products of the reverse reaction.
You may recall that you learned about reaction rate in Unit A when you learned about the collision-activation theory, which explains the events involved in a chemical reaction. How might the probability of collisions between particles and energy of activation influence a chemical system at equilibrium?
In this lesson you will look for evidence of the forward and reverse reactions that occur within chemical systems. By accumulating and interpreting this evidence, you will gain a better understanding of dynamic equilibrium.
Consider the following questions as you complete Lesson 2:
- What is a reverse reaction and why is it important in an equilibrium system?
- What changes occur during dynamic equilibrium?
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
Your lesson assignment consists of a lab. Download a copy of the Module 7: Lesson 2 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. You will also receive further information on how to add to the table you began in Lesson 1.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.8. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore
 Try This
Try This
The Haber process to make ammonia provides an important example of the importance of forward and reverse reactions in a chemical system. Read “The Haber Process” on page 325 in the textbook.
The problems encountered when trying to increase the yield of ammonia are evidence that a reverse reaction is an important aspect of this system. It can occur at the same instant as the forward reaction and in the same reaction vessel.
N2(g) + 3 H2(g) ![]() 2 NH3(g)
2 NH3(g)
In designing a system to maximize the yield of ammonia, experimentation with pressures, temperatures, and catalysts is intended to favour the forward reaction so that large quantities of product can be made. During unsuccessful trials, large quantities were not achieved. In Lesson 6 you will learn more about conditions that may favour the forward or reverse reaction. For now, it is important to understand that both processes exist within a system.
In Lesson 1 you learned that a system at equilibrium is a closed system that has constant properties or does not appear to change. In this lesson you have learned that an equilibrium also involves a forward and a reverse reaction. How do all of these ideas connect? Use the following activity to demonstrate your understanding of these aspects of an equilibrium.
 Try This
Try This
Use the following statement to answer questions TR 1–3: A system at equilibrium is a closed system that has constant properties.
TR 1. Interpret the statement by describing the rate of reaction of the forward and reverse processes for a system that is at equilibrium.
TR 2. Interpret the statement by describing the rates of the forward and reverse reaction for a system that is approaching equilibrium.
TR 3. Provide one observation to support your answer to TR 2.
Save your work in your course folder and submit a copy to your teacher.
 Read
Read
Read page 677 in the textbook to learn more about the different types of equilibria that can exist in chemical systems.
 Self-Check
Self-Check
SC 1. Provide an example of each type of chemical equilibrium described on page 677 of the textbook. Identify the chemical components involved.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. Answers will vary. Some possible answers are shown below.
| Type of Chemical Equilibrium | Example |
Phase Equilibrium |
humidity in air (gaseous water (vapour) and liquid water) |
Solubility Equilibrium |
concentration of calcium and phosphate ions in blood and as a compound in bones and teeth carbon dioxide gas dissolved into the water in a carbonated beverage dissolved oxygen in a body of water |
Chemical Reaction Equilibrium |
ionization of an acid or a base (reaction with water to produce hydronium ions (acid) or hydroxide (base)) |
1.9. Page 3
Module 7—Principles of Chemical Equilibrium
 Module 7: Lesson 2 Lab—Evidence of Reverse Reaction
Module 7: Lesson 2 Lab—Evidence of Reverse Reaction
Purpose
In your chemistry study to date, you have studied reactions that are quantitative. What does a quantitative reaction mean? How does the term quantitative connect with what you are learning about equilibrium? In this virtual investigation, you will observe the results of tests performed on a chemical system to learn more about equilibrium.
As you read the experimental design, you may wish to write out the chemical reactions involved and determine what substance is being tested for using Ba(NO3)2(aq). Contact your teacher if you are having difficulty.
Problem
Can the products of the reverse reaction be detected in a chemical system at equilibrium?
Experimental Design
Four different volumes (25 mL, 50 mL, 75 mL, and 100 mL) of 0.50 mol/L CaCl2(aq) are added to four 50-mL aliquots of 0.50 mol/L Na2SO4(aq). Each system is filtered and the mass of precipitate produced is dried and measured. The filtrate from each system is tested with a saturated solution of Ba(NO3)2(aq).
![]() Retrieve your copy of Module 7: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Answer the Pre-Lab and Hypothesis questions. Record data in the Assignment document as you view the virtual investigation.
Retrieve your copy of Module 7: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Answer the Pre-Lab and Hypothesis questions. Record data in the Assignment document as you view the virtual investigation.
![]() View the virtual investigation “Lab—Evidence of Reverse Reaction.”
View the virtual investigation “Lab—Evidence of Reverse Reaction.”
Analysis
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
You will complete the Analysis in your Assignment document. Complete the Module 7: Lesson 2 Assignment now. Save your completed Assignment in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
1.10. Page 4
Module 7—Principles of Chemical Equilibrium
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you examined a number of chemical systems, and you learned about the forward and reverse reactions that describe each system’s dynamic equilibrium. In Lesson 1 you prepared a table to keep a record of the equilibrium systems you investigate throughout this module.
RBP 1. Add the systems that you looked at in this lesson to your table.
RBP 2. Add two columns to your table. Use the headings “Static or Dynamic Equilibrium?” and “Type of Chemical Equilibrium.” (You may wish to adjust the set-up for the page so that it is landscape, since you will be adding additional columns in future lessons.)
RBP 3. Review all of the systems listed in your table, and complete all empty cells in your table.
Save your updated table in your course folder.
 Module 7: Lesson 2 Assignment
Module 7: Lesson 2 Assignment
Submit your completed Module 7: Lesson 2 Assignment to your teacher.
1.11. Page 5
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following questions:
- What is a reverse reaction and why is it important in an equilibrium system?
- What changes occur during dynamic equilibrium?
You learned that there are three types of equilibrium for chemical systems:
- Phase equilibrium: one chemical substance exists in more than one phase in a closed system.
- Solubility equilibrium: one solute and one solvent exist in a saturated solution that has an excess of solute in a closed system.
- Chemical reaction equilibrium: the reactants create products as fast as the products create reactants; in other words, the forward reaction is approximately equal to the reverse reaction.
Each of these types of equilibria is a dynamic equilibrium, meaning that microscopic changes occur in the system as described by forward and reverse reactions for the chemical components of the system. In this lesson you completed an investigation to test for the products of the reverse reaction. This investigation confirmed that this process occurs, even in conditions involving stoichiometric excess.
1.12. Lesson 3
Module 7—Principles of Chemical Equilibrium
Lesson 3—Position of an Equilibrium
 Get Focused
Get Focused
When you brush your teeth you probably do so to remove harmful plaque that can cause tooth decay. Did you realize that brushing your teeth contributes to an equilibrium system? Most types of toothpaste and dental rinse contain fluoride, an important component in the process of building dental enamel—the outermost tooth layer made of hydroxyapatite and fibrous protein. The layer of enamel is part of a chemical equilibrium involving the processes of enamel formation and erosion. The chemical equilibrium is shown in the reaction
5 Ca2+(aq) + 3 PO43–(aq) + F–(aq) ![]() Ca5(PO4)3F(s)
Ca5(PO4)3F(s)
Can you explain why dentists often ask their patients to use toothpastes and dental rinses containing fluoride? How would you incorporate your understanding of equilibrium to explain this practice?
In Lesson 2 you learned about the three types of chemical equilibria. In Lesson 3 you will study chemical systems in equilibrium in greater depth, and you will investigate the characteristics of these systems.
Consider the following question as you complete Lesson 3:
- Are all equilibrium states similar?
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
In your lesson assignment you will analyze a model of dynamic equilibrium. Download a copy of the Module 7: Lesson 3 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. You will also receive further information on how to add to the table you started in Lesson 1.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.13. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore
 Read
Read
Read the mini investigation “Modelling Dynamic Equilibrium” on page 678 of the textbook.
![]() View the virtual investigation “Mini Investigation: Modelling Dynamic Equilibrium.” As you view the presentation, record the volume of liquid in each cylinder after each transfer of liquid using the straws. Obtain the data showing the results of three other trials for this experiment.
View the virtual investigation “Mini Investigation: Modelling Dynamic Equilibrium.” As you view the presentation, record the volume of liquid in each cylinder after each transfer of liquid using the straws. Obtain the data showing the results of three other trials for this experiment.
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Retrieve your copy of the Module 7: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment.
Save your completed Assignment in your course folder. You will receive instructions later in this lesson on when to submit your work to your teacher.
 Read
Read

© Jack Cronkhite/shutterstock
Did all four systems you examined in “Mini Investigation: Modelling Dynamic Equilibrium” achieve an equilibrium?
As you learned earlier, equilibrium in a chemical system exists when the rates of the forward and reverse reactions equal. As you saw in this investigation, the initial volume of reactant, relative to product, was not important in establishing an equilibrium.
Another important aspect of equilibrium is position of equilibrium. The term equal brings to mind a teeter-totter balancing parallel to the ground. If each side of the teeter-totter represents the concentrations of reactant and products respectively, is a position parallel to the ground the only position possible? If you think about some of the other equilibrium systems you have investigated and summarized in the table you have been keeping, does their equilibrium represent equal concentrations of reactant and products?
In the “Evidence of a Reverse Reaction” lab you completed in Lesson 2, you will recall that the equilibrium in that system involved a great deal of calcium sulfate precipitate and an extremely small quantity of dissociated sulfate ions, which were only detectable by the addition of barium ions. This equilibrium clearly favoured the formation of the products (forward reaction) and resulted in a situation in which the concentration of the products was higher than that of the reactant species.
Systems that significantly favour the formation of products are quantitative systems. You will recall from your work in Chemistry 20 that you were able to perform stoichiometric calculations on chemical systems that had percentage reactions of over 99%. In these systems and relative to the quantity of product, very little reactant remains once equilibrium is established.
Read the section “Chemical Reaction Equilibrium” on pages 678–680 in the textbook to learn more about the different positions of equilibrium in chemical systems.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 3, 4, and 7 on page 682 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 3.
- A system at equilibrium appears not to change, or has a constant set of observable properties.
- An equilibrium involves a forward and a reverse reaction occurring at equal rates. These two reactions involve change at the microscopic level; because change occurs, the system is dynamic.
- The rates of the forward and reverse reactions are equal.
Practice 4.
- CH4(g) + Cl2(g)
 CH3Cl(g) + HCl(g)
CH3Cl(g) + HCl(g)
The maximum possible yield of CH3Cl(g) is 2.00 mol. This is because 2.00 mol of CH4(g) is used and it is the limiting reagent, and all reactants combine in 1:1 molar proportions.
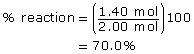
The position of the equilibrium favours the products.
Practice 7.
- Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s)
- <50%
Ca2+(aq) + SO42–(aq) CaSO4(s)
CaSO4(s)
- >50%
CH3COOH(aq) + H2O(l) CH3COO–(aq) + H3O+(aq)
CH3COO–(aq) + H3O+(aq)
1.14. Page 3
Module 7—Principles of Chemical Equilibrium
 Reflect and Connect
Reflect and Connect
The addition of fluoride to tooth care routines has significantly reduced the amount of tooth decay. You will find fluoride in two main sources: in your toothpaste and in your drinking water. In most cities in Alberta, fluoride is added to the drinking water in tiny doses to help fight tooth decay. In some well water, fluoride is found in much greater concentrations than in treated city water. If a little fluoride is helpful to build tooth enamel, then is more better? If you look at the information on a tube of toothpaste, you will see a warning not to ingest your toothpaste. Although a little fluoride can have excellent benefits, too much fluoride can have negative health effects.
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you have examined a number of chemical systems, and you have learned about the forward and reverse reactions that describe their dynamic equilibrium. In Lesson 2 you added to your table of equilibrium systems investigated in this module.
RBP 1. Add the systems that you looked at in this lesson to your table.
RBP 2. Add an additional column to your table. Make the heading for the new column “Position of Equilibrium.”
RBP 3. Review all of the systems listed in your table and complete all empty cells.
Save your updated table in your course folder.
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Submit your completed Module 7: Lesson 3 Assignment to your teacher.
1.15. Page 4
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following question:
- Are all equilibrium states similar?
You completed a simulation of a system demonstrating dynamic equilibrium. This investigation helped you learn more about how an equilibrium can be established and what the relative proportions of products to reactants can be in a system at equilibrium.
You also learned that there are four positions for an equilibrium involving a chemical system:
- Non-spontaneous: these systems have no apparent reaction.
- Products favoured: the equilibrium has a percent yield that is greater than 50%, where the products of the forward reaction are favoured. The outcome for such a system is that the concentration of the products will be higher than that of the reactant species. You need to be able to differentiate this from what occurs when the system reaches its equilibrium and the rates of the forward and reverse reactions in the system are equal.
- Reactants favoured: the equilibrium has a percent yield that is less than 50%, where the reactants of the forward reaction are favoured. The outcome for such a system is that the concentration of the products will be lower than that of the reactant species.
- Quantitative: the equilibrium has a percent yield that is greater than 99%, where the products of the forward reaction are highly favoured.
1.16. Lesson 4
Module 7—Principles of Chemical Equilibrium
Lesson 4—Quantitative Perspectives of Equilibrium
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
In your study of chemical equilibrium so far, you have observed colour changes. In chemical systems in which a coloured substance is involved, colour changes indicate a change in the molar concentration of the coloured substance within the system. Depending on the concentration of one coloured substance relative to other substances, the overall system’s colour describes the position of a system’s equilibrium. If the overall colour changes, it indicates that the equilibrium is changing.
Using colour is not always the best way to describe the position of an equilibrium. In Lesson 3 you determined the position of a chemical equilibrium system using percent reaction. To complete a calculation of percent yield, you require information about the chemical quantities of one reactant and one of the products in the system. If a chemical system involves many components, how can their chemical quantities be determined? What information about the system do these quantities provide?
Determining the quantities of all the reactants and products in a system is an important process in understanding a chemical equilibrium. In Lesson 4 you will learn a systematic procedure to better understand chemical equilibrium from a quantitative perspective.
Consider the following question as you complete Lesson 4:
- What procedures are used to calculate the chemical quantities for all substances in a chemical system?
 Module 7: Lesson 4 Assignment
Module 7: Lesson 4 Assignment
There is no assignment for this lesson. You will be assessed on the material you learn in this lesson as part of the Lesson 5 Assignment.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.17. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore
 Self-Check
Self-Check
In Lesson 3, you completed a series of calculations to determine the percent yield to analyze the following system:
CH4(g) + Cl2(g) ![]() CH3Cl(g) + HCl(g)
CH3Cl(g) + HCl(g)
Initial quantity of CH4(g) = 2.00 mol
Quantity of CH3Cl(g) = 1.40 mol
SC 1. Calculate the quantity of CH4(g) that reacted and that remains unreacted. Explain how you completed these calculations.
SC 2. Recall that 10.00 mol of chlorine were placed into the system initially. Could the process you explained in SC 1 be used to calculate the quantity of Cl2(g) and HCl(g) in the system at equilibrium?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. Mole ratios can be used to complete the calculation for quantity of CH4(g) reacted.
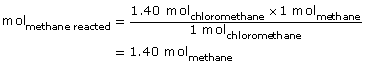
The moles of methane that reacted were 1.40 mol.
The quantity of methane remaining is 2.00 mol − 1.40 mol = 0.60 mol.
SC 2. Using mole ratios will allow the calculation of the remaining substances.
Moles of chlorine reacted will be equal to the moles of methane that reacted, since they combine in 1:1 proportions. Therefore, moles of Cl2(g) reacted = 1.40 mol and moles of Cl2(g) remaining is 10.00 mol –1.40 mol = 8.60 mol.
Moles of HCl(g) produced will be equal to moles of cloromethane produced, since the reactants have 1:1 proportions (as shown in the balanced chemical equation). Therefore, moles of HCl(g) = 1.40 mol.
 Read
Read
The process you described in your answers to SC 1 and SC 2 requires you to apply your knowledge of stoichiometry to a new situation. You know the quantity of one of the products and one of the reactants, and you have a balanced chemical equation. It is possible to use this information and the ratios of coefficients between species to determine the quantities of the other substances in the system.
Using changes in chemical quantities of substances in a system as it approaches equilibrium can be summarized using a structure called an ICE table. To read more about an ICE table and to learn how the calculations you performed are used in its completion, read page 681 in the textbook and work through “Sample problem 15.1.” As you work through the sample problem, note how the stoichiometric ratios are applied to the change in concentration for each species in the system.
 Self-Check
Self-Check
SC 3. What do the letters in ICE represent?
SC 4. Why is the value for the change in the quantity of each species important in completing an ICE table?
SC 5. What does a negative sign in the change row of an ICE table represent?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3. I = initial, C = change, and E = equilibrium concentrations of reactants and products in the equilibrium system.
SC 4. The change in concentration of each species is an important value to have because it identifies the quantity of substance that has undergone chemical change as equilibrium has been established. In a system at equilibrium, some quantity of each species in the system will be present; therefore, the change in quantity for one substance is required to interpret the change in quantity for other substances in the system.
SC 5. A negative sign represents a decrease in quantity for that substance. A decrease in chemical quantity or moles for reactants is expected since reactants are consumed by the forward reaction as the system establishes equilibrium.
1.18. Page 3
Module 7—Principles of Chemical Equilibrium
 Try This
Try This
In this activity you will complete a “Virtual ICE Table.” Use the information provided to determine the values for the empty cells in the table. Enter your answers into the appropriate cells to determine whether your answers are correct.
 Self-Check
Self-Check
SC 6. Complete “Practice” question 6 on page 682 of the textbook.
SC 7. An experiment to study the equilibrium in the following system is conducted:
N2(g) + 3 H2(g) ![]() 2 NH3(g)
2 NH3(g)
In the experiment, 2.42 mol of NH3(g) and 20.76 mol of H2(g) were added to an evacuated 2.00-L flask. At equilibrium the concentration of ammonia in the flask was 1.05 mol/L.
Prepare a table showing the initial, change, and equilibrium concentrations for each of the substances in the system.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 6.
Practice 6.
- The graph shows that the equilibrium concentration of ethene is 2.5 mol/L. The change in ethene concentration is 4.0 mol − 2.5 mol = 1.5 mol. Since the coefficients in the balanced chemical equation are all 1, all species undergo a change in concentration of 1.5 mol/L. Therefore, the equilibrium concentrations are as follows:
Br2(g) = 2.50 mol/L − 1.5 mol/L = 1.0 mol/L
C2H3Br(g) = 0 mol/L + 1.50 mol/L = 1.50 mol/L
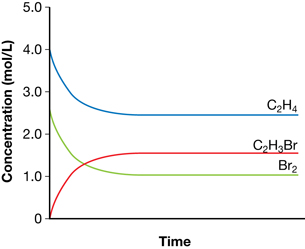
Concentration |
C2H4(g) (mol/L) |
Br2(g) (mol/L) |
C2H3Br(g) (mol/L) |
Initial |
4.0 |
2.5 |
0 |
Change |
–1.5 |
–1.5 |
+1.5 |
Equilibrium |
2.5 |
1.0 |
1.5 |
- The volume of the container is 1 litre. This is determined by the information provided on the graph, which shows an initial concentration of ethane of 4.0 mol/L. Since 4.0 mol of ethane were used, the volume of the container must be 1.0 L.
- Since all coefficients are 1, the moles of bromine are lower than the moles of ethene. Therefore, bromine is the limiting reagent. The maximum yield of bromoethene would be 2.50 mol. Therefore,
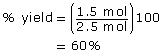
SC 7.
Concentration |
N2(g) (mol/L) |
H2(g) (mol/L) |
NH3(g) (mol/L) |
Initial |
|
|
0 |
Change |
|
|
0 + 1.05 = +1.05 |
Equilibrium |
1.21 − 0.53 = 0.68 |
10.8 − 1.58 = 9.2 |
1.05 |
1.19. Page 4
Module 7—Principles of Chemical Equilibrium
 Reflect and Connect
Reflect and Connect
When taking antibiotics, it is advised that you take the drug at regular time intervals. Can you think of why it is important to take the drug at the times specified?
In order for a drug to have its desired effect, it has to reach a certain concentration in the body. This concentration can be regarded as an equilibrium concentration, since the concentration is a result of the processes of ingestion and absorption of the drug (which will increase the concentration) and the processes of metabolism and excretion (which will decrease the concentration). Can you think of how the processes to reach an equilibrium in the concentration of a medicine in the body are similar to those in the investigation in which you simulated a dynamic equilibrium?
 Module 7: Lesson 4 Assignment
Module 7: Lesson 4 Assignment
There is no assignment for this lesson.
1.20. Page 5
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following question:
- What procedures are used to calculate the chemical quantities for all substances in a chemical system?
You applied your skills in performing stoichiometric calculations to analyze chemical systems in equilibrium. You found that constructing an ICE table is a convenient way to identify the relevant calculations that must be performed to complete this type of analysis.
In Lesson 5 you will learn more about how an equilibrium system can be analyzed using quantitative techniques.
1.21. Lesson 5
Module 7—Principles of Chemical Equilibrium
Lesson 5—The Equilibrium Constant
 Get Focused
Get Focused
© 2008 Jupiterimages Corporation
In Lesson 4 you considered the importance of maintaining the desired concentration of a prescription drug in your system. Maintaining the concentration of a drug in a system involves many equilibria, including the rates at which the drug could be absorbed into your system and the rate at which the drug is metabolized and excreted.
A pharmacologist studies the development and action of drugs in the body. Consideration of equilibria is very important in the work of pharmacologists. Important equilibria to consider in pharmacology include the solubility of the drug and its ionization once in the system. These equilibria influence the ability of the drug to be absorbed by the blood and to have its desired effect in the body. How would a pharmacologist and other scientists study an equilibrium? What sort of information is most useful to them?
In this lesson you will learn how to use numerical data, beyond the percentage reaction calculation performed in previous lessons, to provide information about an equilibrium, including the equilibrium’s position.
Consider the following questions as you complete Lesson 5:
- What is the equilibrium constant?
- What information about an equilibrium is provided in an equilibrium constant?
 Module 7: Lesson 5 Assignment
Module 7: Lesson 5 Assignment
Your assignment will address material covered in Lessons 4 and 5. Download a copy of the Module 7: Lesson 5 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson. You will also receive further information on adding to the table you began in Lesson 1.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.22. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore
In your science and mathematics courses you have come across many “constants.” A constant is a numerical value that describes a relationship between variables within a system. Sometimes constants can have units that help identify the variables they are relating. For example, in previous chemistry courses you were introduced to the universal gas constant, 8.314 L•kPa/(mol•K). You may have even completed an investigation to derive this value from the data you collected.
In the next activity you will derive another familiar constant from your observations—pi.
 Try This
Try This
Derivation of Pi
Materials
- piece of string 30 cm in length
- ruler
- calculator
Step 1: Download the handout “Try This—Circles.”
Step 2: Prepare a data table consisting of four columns and six rows. Label the columns with the following headings: “Circle Number,” “Circumference, C (cm),” “Diameter, d (cm),” “Circumference Divided by Diameter, C/d.”
Step 3: Using the piece of string and the ruler, measure the circumference and diameter of each circle on the handout. Record each value in your data table.
Step 4: Calculate the circumference divided by the diameter for each circle. Record the value obtained for each circle in the appropriate cell of your data table.
Analysis
TR 1. Comment on the results of the calculations for each circle.
TR 2. Do the results demonstrate that a constant relationship exists between the variables of the circles being analyzed?
 Read
Read
The constant pi is used in most calculations involving circle geometry. When completing certain calculations involving gases, you may have used the ideal gas constant or the constants for molar volume of a gas at STP or SATP. Do systems at equilibrium each have a constant? What are the variables that describe an equilibrium? How would these variables be used to calculate a constant?
In your earlier investigations of equilibrium, you used colour changes to monitor whether or not a system was at equilibrium. You will recall that in an equilibrium, all species in the chemical system are present to some extent. Therefore, the equilibrium you observed was a mixture of colours of the reactant and product species.
Depending upon the position of the equilibrium, the colour may have been more like the products or the reactants, but it was always a blending of colours from each group. You also know from your understanding of solutions that colour intensity is directly related to concentration. For example, the higher the concentration of the oxidized form of methylene blue, the more intense the blue colour of the solution in the flask.
From what you have learned so far in this module, you know that some relationships regarding equilibrium are important and need to be considered in any calculation for an equilibrium constant.
Keep the following three important relationships in an equilibrium in mind when calculating an equilibrium constant:
- The concentrations of each species in the system are important values.
- The position of an equilibrium is determined by the relative proportions of products to reactants.
- A balanced chemical equation describes the proportions of reactants and products involved in the system.
1.23. Page 3
Module 7—Principles of Chemical Equilibrium
 Try This
Try This
An Equilibrium Law
Purpose
To test various mathematical formulas to see if they can provide an equilibrium law, a general formula describing how to calculate an equilibrium constant is required.
TR 3. Read “Lab Exercise 15.A” on page 683 of the textbook. Complete an analysis by calculating an answer for each of the proposed formulas for an equilibrium law. Use the trials shown in “Table 6” on page 683. A suitable formula will produce the same value (a constant) from the data for all five trials.
TR 4. Which of the proposed formulas tested in “Lab Exercise 15.A” provides a “constant” for the data analyzed?
Summarize the results of your calculations in a table. Save a copy of your table in your course folder.
 Discuss
Discuss
Post your answer and the table you developed in TR 3 to the discussion area for your class. Use your classmates’ responses to check your skill in accurately completing the calculations and to see whether you can identify a similar relationship that could be used to calculate an equilibrium constant.
Earlier you were asked to consider the three important relationships that should be incorporated into a mathematical approach to calculating an equilibrium constant. To review, these were as follows:
- The concentrations of each species in the system are important values.
- The position of an equilibrium is determined by the relative proportions of products to reactants.
- A balanced chemical equation describes the proportions of reactants and products involved in the system.
D 1. Comment on whether the proposed formula you identified in TR 4 also incorporates the three important relationships in an equilibrium described above.
 Read
Read
Completing the analysis of the data shown in “Lab Exercise 15.A” provides valuable insight into what an equilibrium needs to be. Read from page 684 to the end of the summary on page 686 of the textbook to learn more about the development of an equilibrium law and the equilibrium constant, Kc. Work through the “Communication examples” in this section.
 Try This
Try This
![]() Watch the simulation “The Meaning of the Equilibrium Constant.” You will need your username and password for the textbook. (Remember to ask your teacher if you do not have these.) Once logged in, click on each of the following links in order: Nelson Chemistry Alberta 20-30, Student Web Centre Access, Chapter 15 Equilibrium Systems, Section 15.1, Extensions, simulation (under the header “Extension: The Meaning of the Equilibrium Constant”). The information presented reinforces the information on page 685 in the textbook.
Watch the simulation “The Meaning of the Equilibrium Constant.” You will need your username and password for the textbook. (Remember to ask your teacher if you do not have these.) Once logged in, click on each of the following links in order: Nelson Chemistry Alberta 20-30, Student Web Centre Access, Chapter 15 Equilibrium Systems, Section 15.1, Extensions, simulation (under the header “Extension: The Meaning of the Equilibrium Constant”). The information presented reinforces the information on page 685 in the textbook.
As you work with this simulation, note that you are able to manipulate the following variables:
- value of Kc, the equilibrium constant
- volume of the container
- temperature of the system
- number of spheres in the system
TR 5. As you view the simulation you will be able to see information that describes the numbers of each particle, the speed of particles in the container, the container size, and the concentration of particles of each type versus time. Which of these pieces of information would provide the greatest information about the equilibrium in the system?
TR 6. Prepare a table to record the changes you wish to observe while manipulating each of the variables listed in the bullet list. Record observations of how the equilibrium is affected by the changes you make.
You may wish to complete the self-scoring quiz that appears in the lower box of the animation window. Make note of answers you do not get correct. You may wish to verify your understanding using the textbook or other reference materials.
Save your work in your course folder.
 Read
Read
In some situations, the value for Kc can be used to determine the expected change in the concentration of substances, as equilibrium is established. In such a situation it is important to remember that the value you are required to solve for, is the change in concentration. You may wish to use the structure of an ICE table to help define your variables and relationships. This will help you complete the mathematical calculations.
Read “Predicting Final Equilibrium Concentrations” on pages 686–687 of the textbook. Work through “Sample problem 15.2” in the reading to see how these types of problems are solved.
1.24. Page 4
Module 7—Principles of Chemical Equilibrium
 Self-Check
Self-Check
SC 1. Complete “Section 15.1” questions 1 (a, b, e, f), 2, 4 (a, b), 5, 6, 7, 9, and 10 on pages 688–689 in the textbook.
 Module 7: Lesson 5 Assignment
Module 7: Lesson 5 Assignment
Retrieve your copy of the Module 7: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save your completed Assignment in your course folder and submit a copy to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you have examined a number of chemical systems, and you have learned about the equilibrium law. It's time to add to the table you began in Lesson 1. You last added to your table in Lesson 3.
RBP 1. Add an additional column to your table. Name the column “Equilibrium Expression.”
RBP 2. Write the equilibrium law for each system on the table, or a description of how this would be done.
Save your updated table in your course folder.
1.25. Page 5
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following questions:
- What is the equilibrium constant?
- What information about an equilibrium is provided in an equilibrium constant?
- In this lesson you learned about the equilibrium constant and the equilibrium law. The equilibrium law is an expression that relates the concentrations of the products to the reactants in a chemical system. The equilibrium law is used to calculate an equilibrium constant. The equilibrium law can be stated as
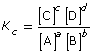 , where the balanced chemical equation for the system takes the form aA + bB
, where the balanced chemical equation for the system takes the form aA + bB  cC +dD
cC +dD
As you found in this lesson, the equilibrium constant is a specific value for a reaction system that is constant for a full range of concentrations. The magnitude of the value for the equilibrium constant provides information about the relative proportions of the products and reactants in the system:
-
if K = 1, then [products] = [reactants]
-
if K > 1, then [products] > [reactants]
-
if K < 1, then [products] < [reactants]
1.26. Lesson 6
Module 7—Principles of Chemical Equilibrium
Lesson 6—Le Châtelier’s Principle
 Get Focused
Get Focused

© Danila/shutterstock
It’s winter and time to get back into the competitive season. The first few races of the snowboarding season are always so tough. Despite training hard in the off-season, snowboarders always feel out of breath and tired when in the mountains.
This kind of experience is common for people who visit altitudes, especially if they exert themselves through strenuous physical activity. You may have noticed that people who live at high altitudes do not seem to suffer from these symptoms. The human body’s ability to adapt to living at altitude has attracted many athletes to live and train in high-altitude locations or to purchase tents that simulate high-altitude conditions.
Since the quantity of oxygen in the air at higher altitudes is reduced as compared to lower altitudes, the human body has to respond to this situation. If you live under these conditions for a prolonged period of time, your body will produce more red blood cells, the blood component responsible for oxygen transport. By producing more red blood cells, your body can capture more of the oxygen from the air, thereby providing your body with sufficient oxygen.
In Lesson 6 you will learn about responses that occur in chemical systems as a result of changes in the conditions they are within. The change to conditions, often referred to as a stress, can change an equilibrium. In this lesson you will investigate whether there is a way to predict the response to a stress placed on a chemical system in an equilibrium.
Consider the following question as you complete Lesson 6:
- What is Le Châtelier’s principle and how is it used to predict changes in chemical equilibrium systems?
 Module 7: Lesson 6 Assignment
Module 7: Lesson 6 Assignment
There is no assignment for this lesson. You will be assessed on the material you learn in this lesson as part of the Module Assessment. Later in this lesson you will be reminded to update the table you began in Lesson 1.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.27. Page 2
Module 7—Principles of Chemical Equilibrium
 Explore
Explore
 Try This
Try This
Demonstration of Equilibrium Shifts
![]() View the virtual investigation “Demonstration of Equilibrium Shifts.”
View the virtual investigation “Demonstration of Equilibrium Shifts.”
Analysis
TR 1. For each of Tubes 2–5, indicate which substance, Co(H2O)62+ or CoCl42–, increases in concentration as a result of the manipulation performed on the tube during the demonstration.
TR 2. Suggest an explanation for the colour changes you observe.
Save a record of your observations and answers in your course folder. Throughout this lesson you will review your observations and consolidate your understanding of the principles introduced and developed throughout Lesson 6.
 Read
Read
In the demonstration you just completed, you saw that changing the conditions for a chemical system equilibrium causes a change to the equilibrium. In the demonstration, a colour change was distinct evidence that a shift in the position of an equilibrium had occurred. In your analysis of the observation, you were asked to identify which coloured species, Co(H2O)62+ or CoCl42, increased in concentration as a result of the change in conditions on that system.
How can the concentration of one of these substances change? Read pages 691–693 in the textbook to begin to address this question.
Le Châtelier’s principle describes a response by a chemical system at equilibrium that has been disturbed. This principle states that when a chemical equilibrium is disturbed, the system seems to respond in the direction that opposes the change (consumes or counteracts the stress). This principle allows you to predict the direction of the shift in the equilibrium.
 Discuss
Discuss
In the virtual investigation “Demonstration of Equilibrium Shifts,” you observed the effect of changing the concentration and temperature on the system being studied. Can you explain the colour change you observed in each of the tubes tested using Le Châtelier’s principle?
D 1. Retrieve your observations and answers to TR 1 and TR 2. Review the changes that occurred in each tube, and write an explanation for the change observed that demonstrates an understanding of Le Châtelier’s principle. Make sure your explanation for the change that occurred in each tube explicitly identifies the following points:
- the “stress” placed on the system
- which reaction, the forward or the reverse, is favoured to respond to the stress
- how favouring the forward or reverse reaction serves to counteract the stress
- the direction of the equilibrium shift
Once you have completed your answers, post them to the discussion area for your class. Review the postings of at least two other students. Provide feedback to the students to help them make their responses more clear and accurate, and to help them ensure they have addressed the aspects listed above.
Revise your response based on the textbook readings and any feedback you receive, and save your revised response in your course folder.
1.28. Page 3
Module 7—Principles of Chemical Equilibrium
 Read
Read
For equilibrium systems containing gases, a change in the volume of the system can also be a stress that will bring about a shift in equilibrium. Read page 694 of the textbook to learn about the effect that a change in volume and the addition of a catalyst can have on the equilibrium of a system. As you read this section you may wish to recall the pressure–volume relationship you learned about in previous science courses—a halving of the volume of a fixed quantity of gas results in a doubling of the gas’ pressure.
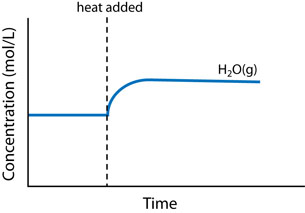
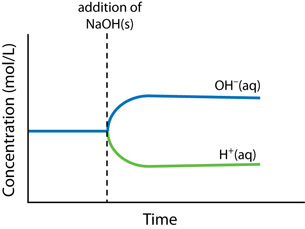
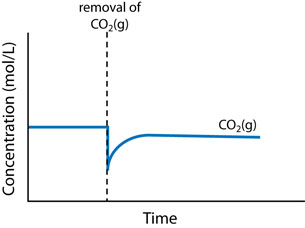
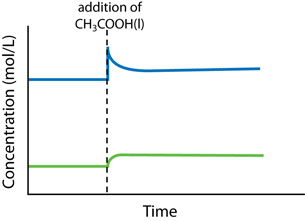
There are three main changes that can affect the equilibrium in a chemical system: concentration change, temperature change, and volume (or pressure) change.
- Concentration change: An increase in concentration causes a shift to consume some of the added reactant or product. A decrease in concentration causes a shift to produce some of the removed reactant or product.
- Temperature change: An increase in temperature of a system causes a shift to absorb some of the added heat energy. A decrease in temperature causes a shift to replace some of the energy that has been removed.
- Volume (or pressure) change: An increase in volume (decrease in pressure) causes a shift toward the side with the larger total chemical amount of gaseous entities. A decrease in volume (increase in pressure) causes a shift toward the side with the smaller total chemical amount of gaseous entities.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 2 and 3 on page 695 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 2.
|
Direction of Shift in Equilibrium |
Graph |
A |
Right |
|
B |
Left |
|
C |
Right |
|
D |
Right |
|
Practice 3.
- Increasing the pressure increases the frequency of collisions between the gaseous particles favouring the forward reaction. The system shifts to the right.
- The forward reaction is exothermic; therefore, increasing the temperature will favour the reverse reaction. The system shifts to the left.
- A high temperature is necessary to speed up the reactions so the process occurs in a reasonable amount of time.
- Both actions, increasing the concentration of reactants and removing the product, are stresses that will favour the forward reaction in the system.
1.29. Page 4
Module 7—Principles of Chemical Equilibrium
 Reflect on the Big Picture
Reflect on the Big Picture
In this lesson you have examined a number of chemical systems and the equilibrium of each one. As you have investigated the systems, you have learned how factors such as pressure, change in concentration, and temperature can act as a stress on these systems. Retrieve the table you began in Lesson 1.
RBP 1. Add two additional columns to your table. Make the headings for the new columns “Equilibrium System Stressed By” and “How Stress Shifts This Equilibrium.”
RBP 2. Complete the empty cells in your table for all of the systems listed.
Save your updated table in your course folder.
 Module 7: Lesson 6 Assignment
Module 7: Lesson 6 Assignment
There is no assignment for this lesson.
1.30. Page 5
Module 7—Principles of Chemical Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 6 you considered the following question:
- What is Le Châtelier’s principle and how is it used to predict changes in chemical equilibrium systems?
Le Châtelier’s principle describes how chemical systems at equilibrium react to stresses such as changes in concentration of species in the system, change to temperature, and changes in the volume (pressure) of the system. Equilibrium systems undergo a shift to a new equilibrium by either favouring the forward or the reverse reaction. In this lesson you learned how to identify a stress placed on a system, the component in the system affected by the stress, and the most likely action by the system to counteract the stress.
Proceed to the Module Assessment where you will have the opportunity to apply your understanding of Le Châtelier’s principle and some of the other aspects of equilibrium you have learned in Module 7.
1.31. Module Summary/Assessment
Module 7—Principles of Chemical Equilibrium
 Module Summary
Module Summary
In Module 7 you considered the following questions:
-
What is happening in a system at equilibrium?
-
How do scientists predict shifts in the equilibrium of a system?
The study of chemical equilibrium has many applications. In this module you learned that chemical equilibrium is an important process in biological and chemical systems. Knowledge of chemical equilibrium is used in many areas, including environmental sciences, chemistry, pharmacology, sports medicine, the chemical industry, and engineering.
In this module you were introduced to the basic concepts of chemical equilibrium and to many applications in which knowledge of equilibrium helps to better understand the function of these systems. You were introduced to many chemical systems that demonstrate principles like position of equilibrium, equilibrium law, and Le Châtelier’s principle.
You have kept track of the systems you investigated in a summary table. Now is a good time to use your table to review the concepts you learned by studying the equilibrium systems. Summaries like the table you constructed throughout this module can really help you prepare for upcoming examinations.
You may wish to continue to use a table like the one you used in Module 7 as you investigate acid and base systems and their equilibria in Module 8. The fundamental aspects of chemical equilibrium you learned in Module 7 will be important for your study in Module 8.
Concept Map or Graphic Organizer
As you worked through Module 7 you may have added information to a concept map or graphic organizer, based on the module and lesson questions listed in the Module 7 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and try to answer the module and lesson questions.
A sample Module 7 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials, and your descriptions, are accurate.
 Module Assessment
Module Assessment
Read the description of the following investigation and view the virtual investigation. Next, complete the Analysis questions. Ensure that your answers include accurate descriptions and explicit reference to the data provided. Remember to state interpretations made from these data using proper representations of, and terminology for, the concepts involved.
Describing a Chemical Equilibrium System
An experiment was performed to investigate the effect of an energy change on the following equilibrium system:
Fe3+(aq) + SCN–(aq) ![]() FeSCN2+(aq)
FeSCN2+(aq)
Experimental Design
A colour change in the substances involved will be used to determine whether a change in equilibrium occurs when a test tube containing a mixture of the components in the system is placed in a warm water bath.
Results
![]() View the virtual investigation “Describing a Chemical Equilibrium System.”
View the virtual investigation “Describing a Chemical Equilibrium System.”
Analysis
Complete the following questions:
- Organize your observations from this investigation into a data table.
- Explain the purpose of test tubes 1, 2, and 3 in the experimental design.
- Does energy affect the equilibrium system investigated?
- Write the chemical equation for this equilibrium system, including the term for energy in the equation.
- What principle was used to investigate the equilibrium in this system? Explain how using this principle allowed you to make interpretations in your answer to question 4.
- Describe one additional experiment that you could perform to confirm your analysis of this eqilibrium system. Include a description of the expected result and an explanation of how the expected result can be used to confirm your analysis.
Save your answers in your course folder and submit a copy to your teacher.
1.32. Module Glossary
Module 7—Principles of Chemical Equilibrium
Module Glossary
closed system: a system that is separated from its surroundings by a definite boundary so that energy can enter and leave the system but matter cannot
dynamic equilibrium: the idea that there is a balance between two opposing processes (forward and reverse) occurring at the same rate
The balance between the forward and reverse processes maintains the system’s appearance of constant properties, in spite of change that has occurred internally.
equilibrium: the state of a closed system in which the system does not appear to change
For a chemical system, physical properties, like colour, remain constant and concentrations of substances within the system do not change.
macroscopic: can be observed with the unaided eye or other senses
microscopic: cannot be observed with the unaided eye or other senses
open system: a system in which both matter and energy can enter and leave the system
static equilibrium: the idea that forces on an object are opposing and equal
The object remains constant because no changes are happening internally.