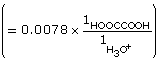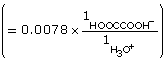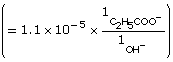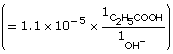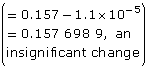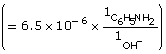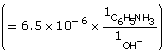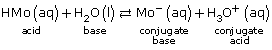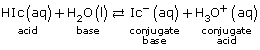Module 8
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 8 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 10:53 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 8
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Lesson 2
- 1.9. Page 2
- 1.10. Page 3
- 1.11. Page 4
- 1.12. Lesson 3
- 1.13. Page 2
- 1.14. Page 3
- 1.15. Page 4
- 1.16. Page 5
- 1.17. Lesson 4
- 1.18. Page 2
- 1.19. Page 3
- 1.20. Page 4
- 1.21. Page 5
- 1.22. Lesson 5
- 1.23. Page 2
- 1.24. Page 3
- 1.25. Page 4
- 1.26. Page 5
- 1.27. Page 6
- 1.28. Lesson 6
- 1.29. Page 2
- 1.30. Page 3
- 1.31. Page 4
- 1.32. Page 5
- 1.33. Lesson 7
- 1.34. Page 2
- 1.35. Page 3
- 1.36. Page 4
- 1.37. Module Summary/Assessment
1. Module 8
Module 8—Acid-Base Equilibrium
© 2008 Jupiterimages Corporation
Module Introduction
In Module 7 you learned about chemical equilibrium. In this module you will apply your understanding of the principles of equilibrium to chemical systems containing aqueous acids and bases. You will use your knowledge of dynamic equilibrium, equilibrium position, and shifts in equilibrium. You will also rely on your ability to analyze an equilibrium system using quantitative techniques (like calculating a value for the equilibrium constant, Kc) and your ability to complete an ICE table.
Your study of the equilibrium of aqueous acids and bases will include an investigation of environmental and biological systems. You will investigate how the components of these systems can interact to form equilibrium systems, and you will consider how the behaviour of these systems can be better understood through knowledge of the principles of chemical equilibrium.
In Module 8 you will investigate the following question:
- How does the equilibrium of acids and bases affect environmental and biological systems?
In Modules 5 and 6 you used a Table of Selected Standard Electrode Potentials to analyze redox systems. The Module 8 Assessment will require you to consider the construction of the Table of Relative Strengths of Acids and Bases, which you will use throughout this module.
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 8, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit D Concept Organizer. In the Module 8 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 8—Acid-Base Equilibrium
 Big Picture
Big Picture

© 2008 Jupiterimages Corporation
Soil is often overlooked when we think of important parts of Earth. In earlier science courses you learned that soil is a component of the biosphere, the section of Earth that supports life. Apart from being the medium for growing most plants, soil is the home for many organisms. Soil is also made up of many chemical components. The minerals, organic materials, and water within soil form a complex chemical system.
The atmosphere also forms a system in which equilibria can exist. How might these equilibria affect the rain and other forms of precipitation that will deposit on Earth?
In Module 7 you learned that certain combinations of chemicals can form an equilibrium system. You also learned that equilibrium systems exhibit certain unique properties that can be manipulated by the application of a stress.
What kinds of stresses are placed on soil? Does human activity or agricultural practice affect the equilibrium of chemical components in soil? How would the chemical components in soil respond to the application of a stress, and what impact might that stress have on soil’s fertility?
Soil is not the only system that has chemical components that form an equilibrium system. In this module you will also investigate the components within biological systems that make up equilibrium systems within organisms.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 8 Assessment you will consider the construction of the Table of Relative Strengths of Acids and Bases, which you will use throughout this module. You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 8—Acid-Base Equilibrium
In This Module
Lesson 1—Introduction to the Equilibrium of Acids and Bases
In Lesson 1 you will be introduced to the equilibrium of acids and bases and the equilibrium of water. You will revisit the concepts of the hydronium ion and of the acidic particle. You will also recall how hydronium ions are formed in aqueous solutions and are believed to be responsible for the properties of acidic solutions.
You will investigate the following lesson questions:
- What is the hydronium ion and how does it account for the properties of acidic solutions?
- What chemical components are part of the equilibrium of water?
- How does the equilibrium of water affect the calculation of pH?
Lesson 2—Acid Strength and Equilibrium
Acids can be classified as either strong acids or weak acids. In Lesson 2 you will apply your understanding of equilibrium to investigate the strength of acids.
You will investigate the following lesson question:
- How is knowledge of equilibrium used to explain the differences in acid strength?
Lesson 3—Brønsted-Lowry Theory
In Lesson 3 you will learn about the development of the Brønsted-Lowry theory. The Brønsted-Lowry theory explains the chemical behaviour of acids and bases.
You will investigate the following lesson questions:
- What occurs during a chemical reaction between an acid and a base?
- How does the Brønsted-Lowry theory support what is known about the equilibrium of aqueous acids and bases?
- How do conjugate acid-base pairs represent an equilibrium system?
Lesson 4—Predicting AB Equilibrium
In Lesson 4 you will test a method to predict the equilibrium position for acid-base reactions.
You will investigate the following lesson question:
- How is knowledge of the equilibrium of aqueous systems involved in predicting the outcome of acid-base reactions?
Lesson 5—Equilibrium Law and the Strength of Acids and Bases
In Lesson 5 you will investigate the use of quantitative techniques to describe the equilibrium of aqueous acids and bases.
You will investigate the following lesson questions:
- What are Ka, Kb, and Kw?
- How do Ka and Kb explain the position of the equilibrium of aqueous acids and bases?
- How are values for Ka and Kb used to calculate the pH of solutions containing weak acids and bases?
Lesson 6—pH Curves
In Lesson 6 you will review acid-base titrations, and you will investigate the parts of a pH curve that demonstrate behaviour as equilibrium systems. You will also learn about acidic and basic substances that can react multiple times and how this process appears on a pH curve.
You will investigate the following lesson questions:
- What information about acids and bases and their equilibrium is contained on a titration curve?
- What events occur in the reaction of polyprotic acids and bases?
Lesson 7—Buffers
In Lesson 7 you will investigate the properties of buffers as equilibrium systems.
You will investigate the following lesson questions:
- What is a buffer and how does a buffer exhibit the characteristics of an equilibrium system?
- What is buffering capacity?
1.3. Lesson 1
Module 8—Acid-Base Equilibrium
Lesson 1—Introduction to the Equilibrium of Acids and Bases
 Get Focused
Get Focused

© Orientaly/shutterstock
Successful farming is not just a matter of planting a crop and seeing what grows. Farming operations cannot control the weather, one key component for success, but they can improve their success by carefully managing the soil.
In the previous module you learned about chemical equilibrium and how chemical systems at equilibrium can be stressed. In this module you will learn about some of the chemical components of soil that are involved in an equilibrium system, and you will discover how some aspects of soil management focus on this equilibrium system.
One aspect of soil management is salinity, which is caused by the concentration of chloride ions in the soil. Soils that experience high saline content can appear to have abundant water, but they cannot support plant growth. In this case, the poor plant growth is due to the effect chloride ions have on the ability of plants to absorb available water. You might think that absorbing water is a simple process, but the movement of water and water's equilibrium in the cells of plant roots and in the soil can be affected by the concentration of chloride ions.
In this lesson you will learn about the equilibrium that exists within water and how this equilibrium is influenced by the presence of acids and bases within an aqueous system.
Consider the following questions as you complete Lesson 1:
- What is the hydronium ion and how does it account for the properties of acidic solutions?
- What chemical components are part of the equilibrium of water?
- How does the equilibrium of water affect the calculation of pH?
 Module 8: Lesson 1 Assignment
Module 8: Lesson 1 Assignment
There is no assignment for this lesson.
There are other questions in this lesson that are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
 Try This
Try This
Summarize What You Know
This module builds on your knowledge of acids and bases gained from previous chemistry courses. You may wish to review the following parts of the textbook to prepare for the remainder of your study in this module:
- “The Nature of Acidic and Basic Solutions,” page 236
- various content about the hydronium ion, page 237
- “pH and pOH Calculations,” pages 238–244
- “Acid–Base Indicators,” page 245
- “The Strength of Acids and Bases,” pages 254–257
- “Explaining Acids and Bases,” pages 248–251
- “Polyprotic Substances,” page 258
- “Titration Analysis,” pages 328–330
- “Acid-Base Titration Curves and Indicators,” pages 333–336
Not in the mood for all that reading? Try entering the word “video” with each of the terms in the bullet list in an Internet search.
Regardless of how you choose to review these concepts, keep a summary of relevant reactions and concepts for each topic. Your summary for each topic might take the form of a list, outline, concept map, or other form. Remember to add to your summaries as you work through this module.
 Read
Read
In Chemistry 20 you learned about the existence of the hydronium ion, H3O+(aq), the acidic particle in aqueous solutions. The hydronium ion is produced by a chemical reaction in which a water molecule becomes protonated. What process allows for a water particle to gain a proton?
You may have hypothesized what would happen if two water molecules were to collide. The resulting reaction would create species that would become part of an equilibrium system within water.
Read pages 713–716 and work through “Communication examples” 1–4 of the textbook to learn more about the equilibrium of water and its equilibrium constant, Kw.
1.5. Page 3
Module 8—Acid-Base Equilibrium
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–4 on page 716 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
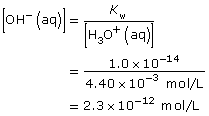
Practice 2.
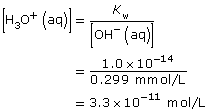
Practice 3.
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq)
Since HCl(aq) is a strong acid, it reacts 100% with water; therefore,
[H3O+(aq)] = [HCl(aq)]
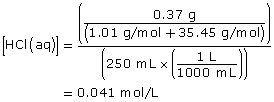
[H3O+(aq)] = 0.041 mol/L
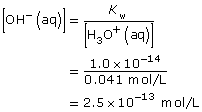
Practice 4.
Ca(OH)2(aq) → Ca2+(aq) + 2 OH–(aq)
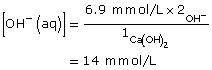
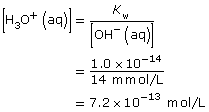
 Discuss
Discuss
In the previous section you used the water ionization constant, Kw, to perform calculations. In Module 7 you learned to interpret the value of equilibrium constants like Kw.
D 1. What does the value for Kw mean about the position of the equilibrium of water?
D 2. Which reaction is favoured in this equilibrium, the forward or reverse, and to what degree?
D 3. What empirical observations do you have to support your answers to D 1 and D 2?
Post your answers to the discussion area for your class, and submit a copy to your teacher.
 Read
Read
Your ability to calculate solution pH and pOH is critical to your investigation of the equilibrium of acids and bases. In Chemistry 20 you were restricted in your ability to perform calculations by not knowing how to convert between concentrations of hydroxide ions and hydronium ions within a solution. With your understanding of the equilibrium of water from the previous section, you can now use Kw to quickly complete such calculations.
Read “Communicating Concentrations: pH and pOH” on pages 716–717 of the textbook. Remember to add new information to the appropriate summary you began at the start of this lesson.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 7–8 on page 718 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 7.
a.
[H3O+(aq)] (mol/L) |
[OH–(aq)] (mol/L) |
pH |
pOH |
|
Oranges |
5.5 × 10–3 |
1.8 × 10–12
|
2.3 (= –log (5.5 × 10–3)) |
11.74 (= 14.00 − 2.26) |
Asparagus |
3 × 10–9
|
3 × 10–6 |
8.4 (= 14.00 − 5.6) |
5.6 |
Olives |
5 × 10–4
|
2.0 × 10–11 |
3.30 |
10.70 (= 14.00 − 3.30) |
Blackberries |
4 × 10–4 (=10–3.4) |
3 × 10–11 |
3.4 (= 14.00 – 10.6) |
10.6 |
b. The oranges would taste most sour because they have the lowest pH.
Practice 8.
NaOH(aq) → Na+(aq) + OH–(aq)
Since NaOH(aq) is highly soluble in water, it completely dissociates. Therefore,
[OH–(aq)] = [NaOH(aq)]
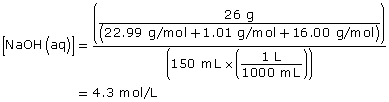
[OH–(aq)] = 4.3 mol/L
![]()
![]()
1.6. Page 4
Module 8—Acid-Base Equilibrium

© AndreyTTL/shutterstock
 Reflect and Connect
Reflect and Connect
Earlier in this lesson you learned that the salinity of soil affects plants’ ability to absorb water. In soils with high salinity, the large concentration of chloride ions in the soil retains water, preventing the water from being absorbed by plants.
Soil salinity is the result of the leaching of chloride ions from salt formations beneath the surface and accumulations of the solutes where water collects and evaporates. In areas with high salinity, like the one shown in the photograph, a solubility equilibrium exists where salt precipitates and dissolves depending on the availability of water.
![]() Complete the following investigation to analyze the solubility of chloride ions in the equilibrium provided.
Complete the following investigation to analyze the solubility of chloride ions in the equilibrium provided.
Read “Exploration: Salty Acid or Acidic Salt?” on page 711 of the textbook. Then watch the virtual presentation “Exploration: Salty Acid or Acidic Salt?”
 Self-Check
Self-Check
SC 3. Complete “Exploration: Salty Acid or Acidic Salt?” questions a–f on page 711 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
- Both solutions, sodium chloride and hydrochloric acid, contain dissociated chloride ions. Therefore, there will be no reaction when the two solutions are mixed.
- NaCl(aq)
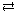 Na+(aq) + Cl–(aq)
Na+(aq) + Cl–(aq)
- Kc = [Na+(aq)] [Cl–(aq)]. Since the sodium chloride solution is saturated, all substances in the system have a constant concentration.
- When the concentrated hydrochloric acid was added, a white precipitate formed. It appears that the addition of HCl(aq) shifts the equilibrium to the left, favouring the reverse reaction and allowing the formation of NaCl(s).
- The addition of HCl(aq) adds Cl–(aq) and some water to the system but does not add Na+(aq). Therefore, the concentration of sodium ions decreases when HCl(aq) is added. This could be a stress on the system and, as predicted by Le Châtellier’s principle, the forward reaction would be favoured (to increase the concentration of Na+(aq)).
The result of this stress is a further increase in the concentration of chloride ions to a level that exceeds the concentration that is possible to remain dissolved. This situation favours the reverse reaction, and the precipitate of NaCl(s) forms.
- The concentration of chloride ions in the concentrated hydrochloric acid must have been higher than the concentration of chloride ions in the saturated sodium chloride solution. Any other possibility would not have resulted in a situation that increased the concentration of Cl–(aq) past its maximum solubility.
 Reflect and Connect
Reflect and Connect
You initiated summaries earlier in this lesson. Update your summaries to include concepts you learned in this lesson. Save a copy of your updated summaries in your course folder. You may be asked to submit a copy of your summaries to your teacher or to share one or more of your summaries with other students in your class.
 Module 8: Lesson 1 Assignment
Module 8: Lesson 1 Assignment
There is no assignment for this lesson.
1.7. Page 5
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- What is the hydronium ion and how does it account for the properties of acidic solutions?
- What chemical components are part of the equilibrium of water?
- How does the equilibrium of water affect the calculation of pH?
You learned that pure water is a chemical system with an equilibrium between hydronium ions and hydroxide ions, albeit at extremely low concentrations. You used the water ionization constant to calculate the molar concentrations of [H3O+(aq)] and [OH–(aq)] that exist within water as a result of water’s equilibrium. You also reviewed the calculation of pH and pOH, further incorporating your knowledge of the equilibrium of water.
1.8. Lesson 2
Module 8—Acid-Base Equilibrium
Lesson 2—Acid Strength and Equilibrium

© 2008 Jupiterimages Corporation
 Get Focused
Get Focused
Acid rain is an environmental issue that has received much attention. However, rain is only one mechanism by which acidic material from the atmosphere can meet Earth’s surface. Acid deposition is a broader term that refers to all forms of wet and dry deposits that have acidic properties. How can rain, snow, and even dust become acidic? Can you recall the chemical reaction that produces the acidic particle, the hydronium ion, and explains the properties of acids?
Four acids have been implicated in acid deposition. The four acids include nitrous and nitric acids, from emissions of nitrous oxides, and sulfurous and sulfuric acids, from emissions of sulfur oxides. Are there any differences in properties among these four acids? Which of these acids poses the greatest risk to the environment?
In your previous chemistry course you learned about the concept of weak and strong acids. How does this classification relate to the chemical reaction described above and its equilibrium? Which of the four acids are categorized as strong? Which are categorized as weak? How might the classification of these substances explain their effects on the environment?
Consider the following question as you complete Lesson 2:
- How is knowledge of equilibrium used to explain the differences in acid strength?
 Module 8: Lesson 2 Assignment
Module 8: Lesson 2 Assignment
Download a copy of the Module 8: Lesson 2 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.9. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
 Read
Read
In Module 7 you used information about the extent of reaction to understand equilibrium position. Another way to understand equilibrium position is to observe the properties of a system. When considering acids, you learned that strong acids react (ionize) 100% with water, whereas weak acids react (ionize) less than 100% with water. Which properties of an acidic solution would be affected by the differences in the extent of reaction (ionization)?
Read “Acid Strength as an Equilibrium Position” on pages 718–720 of the textbook. Work through “Communication example 5” and “Communication example 6” on page 720 to learn how percent ionization is used in the calculation of pH and how a pH value can be used to determine a percent ionization for an acid.
 Self-Check
Self-Check
Complete “Section 16.1” questions 1, 2, 4, 6, and 7 on page 721 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
Section 16.1 1.
- If a solution is neutral, then
[H3O+(aq)] = [OH–(aq)]
- If a solution is acidic, then
[H3O+(aq)] > [OH–(aq)]
- If a solution is basic, then
[H3O+(aq)] < [OH–(aq)]
Section 16.1 2.
Conductivity: This test would detect the degree of ionization. Strong acids will ionize to a greater extent than weak acids and, therefore, would produce solutions with a higher conductivity.
pH: Since strong acids ionize to a greater extent than weak acids, solutions of equimolar solute concentrations of strong acids will have lower pHs.
Section 16.1 4.
- HCN(aq) + H2O(l)
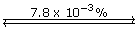 H3O+(aq) + CN–(aq)
H3O+(aq) + CN–(aq)
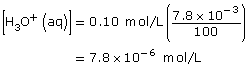
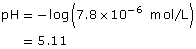
Section 16.1 6.
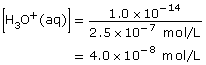
![]()
Section 16.1 7.
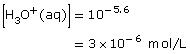

© 2008 Jupiterimages Corporation
 Discuss
Discuss
In the previous Read section you learned that the distinction between a strong and a weak acid is the extent to which the acid ionizes. How does this definition fit with your understanding of an equilibrium system?
Hydrochloric acid is a strong acid, whereas hydrofluoric acid is a weak acid. Use a balanced chemical equation to describe the equilibrium position of these two different types of acids.
Post a copy of your response to the discussion area for your class. Save a copy of your response in your course folder and submit a copy to your teacher.
1.10. Page 3
Module 8—Acid-Base Equilibrium
 Reflect on the Big Picture
Reflect on the Big Picture
Think back to the Module 8 Big Picture, which discussed examples of equilibrium in the soil, water, and atmosphere.
The combustion process that occurs in automobile engines and furnaces produces nitrous oxides, NO(g) and NO2(g). The following unbalanced series of reactions describes the deposition of nitric acid as a result of the chemical joining of nitrogen and oxygen that occurs during a combustion reaction.
N2(g) + O2(g) + 180 kJ ![]() 2 NO
2 NO ![]() NO2(g)
NO2(g) ![]() HNO3(g) → deposition
HNO3(g) → deposition
Use your knowledge of equilibrium to explain how the energy from high-temperature combustion processes contributes to the production of HNO3(g).
Save a copy of your response in your course folder. You will further evaluate your answer later in this module.
 Reflect and Connect
Reflect and Connect
In Lesson 1 you initiated a summary about the strength of acids and bases. Take this opportunity to update your summary to include concepts you learned in this lesson. Save a copy of your updated summary in your course folder. You may be asked to submit a copy of this summary to your teacher for feedback.
 Module 8: Lesson 2 Assignment
Module 8: Lesson 2 Assignment
Retrieve your copy of the Module 8: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save a copy of your completed Assignment in your course folder and submit a copy to your teacher.
1.11. Page 4
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following question:
- How is knowledge of equilibrium used to explain the differences in acid strength?
Acids can be classified as either strong or weak as a result of their ionization, which produces hydronium ions. Strong acids ionize completely. You can consider the concentration of hydronium ions that results from a strong acid’s ionization to be equal to the initial concentration of the acid present in the solution. Weak acids ionize to a small extent. In the case of weak acids, the extent of ionization must be determined or known in order to predict the resulting concentration of hydronium ions.
Knowledge of equilibrium allows for a description of the ionization of strong and weak acids and for a calculation of the acid’s percentage ionization. In Lesson 5 you will further study the equilibrium of strong and weak acids using the equilibrium lab and the ionization constant for acids.
1.12. Lesson 3
Module 8—Acid-Base Equilibrium
Lesson 3—Brønsted-Lowry Theory

© 2008 Jupiterimages Corporation
 Get Focused
Get Focused
The concept of acids you currently work with involves a reaction with water to produce hydronium ions. As you know from earlier in this module, bases dissociate to produce hydroxide ions. Those descriptions work well for substances like hydrochloric acid and calcium hydroxide, but how do you explain the acidic properties of the ammonium ion and the basic properties of the carbonate ion?
Ammonium is commonly used in nitrogen-based fertilizers, and carbonate is one of many chemical components in soil—especially soils in Alberta. What is necessary to better understand the acidic and basic properties of these substances? Would this understanding help you to predict changes that might occur to soil as a result of soil management?
Consider the following questions as you complete Lesson 3:
- What occurs during a chemical reaction between an acid and a base?
- How does the Brønsted-Lowry theory support what is known about the equilibrium of aqueous acids and bases?
- How do conjugate acid-base pairs form an equilibrium system?
 Module 8: Lesson 3 Assignment
Module 8: Lesson 3 Assignment
There is no assignment for this lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.13. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
At least one of the summaries you started in Lesson 1 will have covered early theories for acids and bases. The early theories proposed that dissociation of solutes to produce hydrogen ions or hydroxide ions could explain the acidic and basic properties of solutions. A flaw of these theories was the lack of an ability to explain the properties of common substances that clearly have acidic or basic properties.
- A modification to these early theories required the consideration of the possibility of a chemical reaction between the solute and water. For example, the reaction between aqueous hydrogen chloride, HCl(aq), with water is
- HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq)
The product of this reaction is the hydronium ion; it was later confirmed to be the acidic particle.
- The reaction of a substance like ammonia, NH3, with water is
- NH3(aq) + H2O(l) → NH4+(aq) + OH–(aq)
The earlier theory could not explain the basic properties of aqueous ammonia, but the reaction with water shows the probability of hydroxide ions being produced as products of the reaction. Simple diagnostic tests, like litmus, confirm this theoretical explanation.
 Discuss
Discuss
In previous lessons you considered each reaction as an equilibrium, and you used methods to communicate equilibrium position. Consider the following systems:
System |
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq) |
Concentration of HCl(aq) |
0.0250 mol/L |
Solution pH |
1.6 |
System |
NH3(aq) + H2O(l) → NH4+(aq) + OH–(aq) |
Concentration of NH3(aq) |
0.0250 mol/L |
Solution pH |
10.8 |
D 1. Explain how you would use the information provided to determine the equilibrium positions for the two systems. Give reasons for your suggested approach.
Post your proposed strategy to the discussion area for your class. Read the strategies of at least two other students. How are the other students using the data provided? Will their strategies work? Provide constructive feedback to your classmates regarding the strategies they posted. Revise your strategy based on your readings and the feedback you receive. Make sure you record the reason for any modifications made. Save your work in your course folder.
D 2. Use the information provided to determine and appropriately communicate the equilibrium position for the reactions shown.
Save a copy of your answers in your course folder and submit a copy to your teacher.
 Read
Read
You might conclude that it appears that many substances require a reaction with water to exhibit their properties. Do any general trends exist in these reactions?
Read pages 722–724 in the textbook to examine the trends that might exist in the reactions of acids and bases.
Brønsted-Lowry Concept
In the textbook reading you learned that the Brønsted-Lowry concept redefines how you will consider acids and bases as entities in a chemical reaction. According to this theory you will describe acids and bases as a result of the kind of chemical change they undergo, rather than by their empirical properties.
Because you have seen that some substances have the ability to react as either an acid or a base, you will further modify your concept of acids and bases. Later in this module you will learn how to predict when these substances will act in one of these possible roles.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–5 on page 724 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
- According to the Arrhenius theory, acids are substances that ionize in water to form aqueous hydrogen ions.
- According to the modified Arrhenius theory, acids are substances that react with water to form aqueous hydronium ions.
- According to the Brønsted-Lowry concept, acids are substances that donate a hydrogen ion (proton) during chemical reactions.
Practice 2.
Both definitions predict the hydroxide ions as a product of the processes involved but differ in explaining how the hydroxide ion is produced. The Arrhenius theory explains the ion's production as a result of dissociation of the solute. The Brønsted-Lowry concept requires that a substance react with water; in the resulting transfer of a proton from the water molecule to the solute, a hydroxide ion is produced.
Practice 3.
- HF(aq) acid, SO32–(aq) base
- CO32–(aq) base, CH3COOH(aq) acid
- H3PO4(aq) acid, OCl–(aq) base
- HCO3–(aq) base, HSO4–(aq) acid
Practice 4.
- HSO3–(aq) + OH–(aq)
 SO32–(aq) + H2O(l)
SO32–(aq) + H2O(l)
acid base
- HSO3–(aq) + H3O+(aq)
 H2SO3(aq) + H2O(l)
H2SO3(aq) + H2O(l)
base acid
Practice 5.
The Brønsted-Lowry concept allows for the ability to describe the reaction between acids and bases and to explain how some substances could behave as amphiprotic species.
1.14. Page 3
Module 8—Acid-Base Equilibrium
 Read
Read
The proton transfer reaction during acid-base reactions, as described by the Brønsted-Lowry concept, is unique. For instance, the reaction of ammonia, NH3(aq), produces the ammonium ion, NH4+(aq). Note that the difference between these two chemical formulas is one proton. More interesting is that it is foreseeable that the product could easily donate a proton in a future reaction, thus acting as a Brønsted-Lowry acid.
Read “Conjugate Acids and Bases” on pages 724–726 in the textbook to learn more about acid-base pairs.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 7–8 on page 726 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 7.
- HCO3–(aq), CO32–(aq) and S2–(aq), HS–(aq)
- H2CO3(aq), HCO3–(aq) and OH–(aq), H2O(l)
- HSO4–(aq), SO42–(aq) and HPO42–(aq), H2PO4–(aq)
- H2O(l), H3O+(aq) and H2O(l), OH–(aq)
Practice 8.
HCO3–(aq), CO32–(aq) and H2CO3(aq), HCO3–(aq)
 Try This
Try This
In the previous section you learned about the proton transfer concept. In this activity you will further test this theory by applying material learned in this and previous lessons.
TR 1. The following acids are responsible for acid deposition. Can their acidity be explained using the proton transfer concept?
- HNO3(aq)
- HNO2(aq)
- H2SO4(aq)
- H2SO3(aq)
TR 2. Carbonate ions, CO32–, from limestone, the mineral calcium carbonate, are a component of soils in Alberta. Soils in Alberta tend to be alkaline, as does the pH of the water in most Alberta lakes. Is it possible that the observed pH of soil and lake water could be due to the presence of carbonate in soil?
TR 3. Acid deposition has had relatively little effect on the pH of soils in most parts of Alberta. Use a chemical reaction to explain this observation. In the reaction you write, identify the conjugate acid-base pairs.
Submit your answers to your teacher for feedback.
1.15. Page 4
Module 8—Acid-Base Equilibrium
 Reflect and Connect
Reflect and Connect
In Lesson 1 you started summaries about your previous learning on acids and bases. Take this opportunity to update the appropriate summary to include concepts you learned in this lesson. Save a copy of your updated summary in your course folder. You may be asked to submit a copy to your teacher for feedback.
 Module 8: Lesson 3 Assignment
Module 8: Lesson 3 Assignment
There is no assignment for this lesson.
1.16. Page 5
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- What occurs during a chemical reaction between an acid and a base?
- How does the Brønsted-Lowry theory support what is known about the equilibrium of aqueous acids and bases?
- How do conjugate acid-base pairs form an equilibrium system?
The Brønsted-Lowry concept states that acids act as proton donors and bases act as proton acceptors during chemical reactions. This concept explains the observations of acidic or basic properties of substances that previous theories could not explain.
Further empirical evidence to support this concept comes from the changes to pH that occur during some chemical reactions. These changes can only be explained if it is assumed that a proton transfer has occurred between reacting species.
Finally, you learned to consider a Brønsted-Lowry reaction in terms of conjugate pairs of acids and bases, and you learned about the potential of these systems to act in equilibrium.
1.17. Lesson 4
Module 8—Acid-Base Equilibrium
Lesson 4—Predicting AB Equilibrium

© Pichugin Dmitry/shutterstock
 Get Focused
Get Focused
In Lesson 3 you learned about the Brønsted-Lowry concept. You were able to use this principle to explain the chemical change occurring in an acid-base reaction. However, this principle is not able to explain why certain acid-base combinations will react and others will not. In this lesson you will investigate how to predict the outcome of acid-base reactions and to determine the equilibrium position for such reactions.
In the previous lesson you investigated the reaction between carbonate ions found in soil and nitric acid, which is responsible for acid deposition. You were able to identify this reaction that might neutralize the nitric acid. However, with your current knowledge, you have no way to know whether the reaction between the two components will occur when the components are mixed.
Consider the following question as you complete Lesson 4:
- How is knowledge of the equilibrium of aqueous systems involved in predicting the outcome of acid-base reactions?
In this lesson you will begin to use the “Relative Strengths of Acids and Bases at 298.15 K” table located on pages 8 and 9 of the Chemistry Data Booklet. You may recall that the Module 8 Assessment requires you to consider the structure of this table. It may be helpful to review the Module 8 Assessment before you start this lesson.
 Module 8: Lesson 4 Assignment
Module 8: Lesson 4 Assignment
In this lesson you will complete the lab “Predicting Acid-Base Reactions.” Download a copy of the Module 8: Lesson 4 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all of the questions and place those answers in your course folder.
1.18. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
 Read
Read
Read the first paragraph of “Predicting Acid-Base Reaction Equilibria” on page 727 of the textbook.
Empirical data is necessary to develop general trends about the tendency of acids and bases to transfer protons. Review the “Relative Strengths of Acids and Bases at 298.15 K” table located on pages 8 and 9 of the Chemistry Data Booklet. This table is also available on page 829 of the textbook. A few points about the structure of this table in the Chemistry Data Booklet are as follows:
- Columns 1 and 2 list the name and chemical formula for acids. Column 3 contains the chemical formula of the conjugate base for the acid shown in each row.
- Acids are listed by decreasing strength. For example, perchloric acid is a stronger acid than citric acid; as a result, citric acid is listed further down the table.
- The acids listed above the shaded hydronium ion row are strong acids; acids listed below the hydronium ion row are weak acids.
- Bases are listed by increasing strength, with the strongest base being hydroxide. The remaining substances listed in the conjugate base column are weak bases and are listed by increasing strength. For example, the perchlorate ion, ClO4–(aq), is the weakest base on the table. Substances listed below the perchlorate ion demonstrate increasing strength as bases.
 Try This
Try This
Predicting Acid-Base Reactions
Earlier in this course you used the “Table of Selected Standard Electrode Potentials,” found on page 7 of the Chemistry Data Booklet, to predict and analyze reduction-oxidation reactions. Can the “Relative Strengths of Acids and Bases at 298.15 K” table be used to predict acid-base reactions in a similar way? Read “Lab Exercise 16.B” on page 727 of the textbook to test this possibility.
In “Lab Exercise 16.B,” examine the positions of the substances involved in reactions 1–6. Note the position of the equilibrium for each of these reactions. Comment on any general trends you see.
Save your comments in your course folder and submit a copy to your teacher for feedback.
 Read
Read
Read “Predicting Acid-Base Reactions” on pages 728–731 of the textbook to confirm the trends you may have observed in “Lab Exercise 16.B.”
Predicting the equilibrium position for acid-base reactions involves identifying reacting species and their positions on the “Relative Strengths of Acids and Bases at 298.15 K” table with greater specificity than when you predicted redox reactions.
To successfully predict acid-base reactions, you must list the reaction substances. Already in this module you have learned that the extent to which strong and weak acids react with water differs. With this in mind, what is the most appropriate way to list the species present in a solution of hydrochloric acid?
The following chart summarizes the way substances should be considered when predicting acid-base reactions and the reason for each guideline:
Listing for Substance |
Reason |
All strong acids are written as H3O+(aq). |
Strong acids ionize 100% in aqueous solution; therefore, unionized acid particles are not present except at extremely low concentrations. |
Strong bases (hydroxides) are listed as |
Ionic hydroxide compounds will dissociate to produce OH–(aq). |
Ionic compounds are listed as dissociated ions. |
Ionic compounds dissociate to some extent in water. |
Molecular substances are written in their molecular form; this includes weak acids and weak bases. |
The extent of reaction with water is uncertain; therefore, substances are listed in a unionized form. |
Metallic ions may be omitted from consideration as possible reactants. |
Metallic ions do not have a proton to donate and they do not demonstrate the ability to accept a proton during a chemical reaction. |
 Self-Check
Self-Check
SC 1. Complete the following chart, which lists the equilibrium position for reactions occurring between substances shown on the “Relative Strengths of Acids and Bases at 298.15 K” table.
Reactants/Position of Reactants |
Description of Equilibrium Position |
Symbol Used |
Hydronium and Hydroxide |
|
|
Acid Above Base |
|
|
Base Above Acid |
|
|
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Reactants/Position of Reactants |
Description of Equilibrium Position |
Symbol Used |
Hydronium and Hydroxide |
quantitative |
|
Acid Above Base |
products favoured (greater than 50%) |
|
Base Above Acid |
reactants favoured (less than 50%) |
|
1.19. Page 3
Module 8—Acid-Base Equilibrium
 Read
Read
To practise the method of predicting acid-base reactions, work through “Sample problem 16.1” and “Communication example” on pages 729 and 730 of the textbook. You may wish to listen to the “Sample Problem 16.1 Audio Recording.”
![]() For more practice, work through the animated sample problem “Predicting Acid-Base Reactions.”
For more practice, work through the animated sample problem “Predicting Acid-Base Reactions.”
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 9–15 on page 731 of the textbook.
 Module 8: Lesson 4 Lab—Testing Brønsted-Lowry Reaction Predictions
Module 8: Lesson 4 Lab—Testing Brønsted-Lowry Reaction Predictions
In this lesson you have learned a method to predict acid-base reactions and their equilibrium position. It is important that all theoretical methods like this one be tested.
![]() Retrieve your copy of the Module 8: Lesson 4 Assignment you saved to your computer earlier in this lesson. Complete the Pre-Lab section of the Assignment. As you view the virtual investigation, record your observations in the appropriate section of your Lesson 4 Assignment.
Retrieve your copy of the Module 8: Lesson 4 Assignment you saved to your computer earlier in this lesson. Complete the Pre-Lab section of the Assignment. As you view the virtual investigation, record your observations in the appropriate section of your Lesson 4 Assignment.
![]() View the virtual investigation “Testing Brønsted-Lowry Reaction Predictions.”
View the virtual investigation “Testing Brønsted-Lowry Reaction Predictions.”
 Module 8: Lesson 4 Assignment
Module 8: Lesson 4 Assignment
Complete the Analysis section of the Lesson 4 Assignment.
Save your work in your course folder. You will receive instructions later in this lesson on when to submit your Assignment to your teacher.
1.20. Page 4
Module 8—Acid-Base Equilibrium
 Reflect and Connect
Reflect and Connect
At the beginning of this lesson you were asked whether the proposed reaction between nitric acid in acid deposition would be neutralized by carbonate ions present in soil.
Use your understanding of predicting acid-base reactions and equilibrium position to determine whether nitric acid and the other acids responsible for acid deposition (nitrous, sulfuric, and sulfurous acids) would also be neutralized by carbonate ions present in soil.
Save your work in your course folder.
In Lesson 1 you started summaries of your previous learning about acids and bases. Update your summaries to include concepts you learned in this lesson. You may wish to start a new summary for the new skill you learned in this lesson—predicting acid-base reactions. Save a copy of your updated summaries in your course folder. You may be asked to submit a copy to your teacher for feedback.
 Module 8: Lesson 4 Assignment
Module 8: Lesson 4 Assignment
Submit your completed Module 8: Lesson 4 Assignment to your teacher.
1.21. Page 5
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following question:
- How is knowledge of the equilibrium of aqueous systems involved in predicting the outcome of acid-base reactions?
The focus of this lesson has been on developing and testing a method to predict acid-base reactions and the position of their equilibrium. You learned the principles upon which this method of prediction is based, and you tested predictions by completing a virtual investigation. You also considered how an understanding of this method could be applied to investigating the action of soil components against acid deposition.
Knowledge of this method is required to complete the rest of Module 8. In the next few lessons you will further your understanding of acid-base equilibrium by investigating the chemical reaction occurring in a titration and the composition and action of chemical buffers.
1.22. Lesson 5
Module 8—Acid-Base Equilibrium
Lesson 5—Equilibrium Law and the Strength of Acids and Bases

© Christina Richards/shutterstock
 Get Focused
Get Focused
The pH of solutions provides important information about the equilibrium of acidic and basic solutes. In the previous lessons you used solution pH and molar concentration of the solute to calculate a percent ionization. In Module 7 you learned about equilibrium law and Kc, the equilibrium constant. Can Kc be calculated for acidic and basic solutions? What would a difference in the value for the equilibrium constant for two acids or bases tell you about their equilibrium?
You may recall reading in the textbook that the pH of rainwater is normally 5.5. The presence of dissolved carbon dioxide from the atmosphere in the rain droplets results in the formation of carbonic acid, which in turn ionizes to produce hydronium ions. Why is the pH of rainwater expected to be 5.5?
In this lesson you will investigate the use of quantitative techniques to describe the equilibrium of aqueous acids and bases. You will also investigate how the equilibrium influences the pH of both strong and weak acids and bases.
Consider the following questions as you complete Lesson 5:
- What are Ka, Kb, and Kw?
- How do Ka and Kb explain the position of the equilibrium of aqueous acids and bases?
- How are values for Ka and Kb used to calculate the pH of solutions containing weak acids and bases?
 Module 8: Lesson 5 Assignment
Module 8: Lesson 5 Assignment
There is no assignment for this lesson. Because of the nature of this lesson, the questions that are not marked by the teacher are very important. You should make an effort to ensure that you are confident with the calculations necessary to complete the work in this lesson before you proceed to Lesson 6.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.23. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
Your work in Lessons 3 and 4 has helped you add to your knowledge of acids and bases. Because of your work to understand the Brønsted-Lowry theory, you probably find it easier to define acids and bases by what they do in a reaction rather than by their empirical properties.
It is often easy to confuse the term strength with concentration when talking about acids and bases. Strength refers to the extent to which a substance ionizes. For instance, strong acids react 100% to produce hydronium ions. Concentration refers to the chemical amount of solute per unit volume of solution. It is possible to have a highly concentrated solution in which a weak acid is the solute.
In Module 7 your exploration of chemical equilibrium involved learning about percent reaction and the equilibrium constant for a system. As you have seen through your studies so far, both values are commonly used to describe the equilibrium position. You may wish to test your memory and write the formulas to calculate percent reaction and the equilibrium constant, and then adapt your calculations for an aqueous acid. How does the similar position of the term [H3O+(aq)] in both relationships account for the ability to use values for percent reaction and the equilibrium constant to describe equilibrium position?
 Read
Read
What would the equilibrium law expression and the equilibrium constant for acidic and basic systems look like? Read pages 737–738 in the textbook to find out. In your readings note that the equilibrium law expression for an acid is slightly different that what you may recall from Module 7. What assumption is being made in order to make this modification?
You may find the “Learning Tip” on page 738 useful in helping you distinguish between the function of a value for percent ionization and a value for Ka, the ionization constant for an acid.
 Try This
Try This
Work through the “Sample problems” and “Communication examples” on pages 738–741 of the textbook to learn how to calculate Ka.
TR 1. Can you explain why an ICE table was used to solve these problems?
 Read
Read
In Module 7 you learned that ICE tables are an effective way to represent the changes that occur within a chemical system as the system moves from its initial set-up to equilibrium. You will recall that you only use equilibrium concentrations to calculate the value of the equilibrium constant, whether it be a Kc or a Ka. Completing an ICE table is a good way to sort out the changes that are occurring in a system moving to an equilibrium since, in the action of the forward reaction, reactants will be consumed to make products.
The rows in the ICE table represent the change in the concentration and the equilibrium concentration of each species. The information in these rows are reminders of the chemical processes that occur within the system when components mix and react as the system establishes an equilibrium.
You may notice that, in some cases (e.g., “Sample problem 16.2” and “Communication example 1” on pages 738–739), the concentration of hydronium ions produced as the acid ionizes is quite small. If you look at the first column of the ICE tables in these two textbook examples, you will notice that the “change in concentration” for the acid is so small that the value for the final and initial concentrations are the same. This inaccuracy affects the value for Ka and is the reason that values for Ka, like those that appear in the Chemistry Data Booklet, are often only shown with two significant digits.
As you see in “Communication example 2” on page 739, you cannot always assume that the change in initial concentration of the acid will be small. Therefore, it is a good practice to construct an ICE table when solving these types of problems. Doing so will ensure your calculations have the required accuracy and will demonstrate your complete understanding of the changes that occur as a system develops an equilibrium.
1.24. Page 3
Module 8—Acid-Base Equilibrium
 Self-Check
Self-Check
SC 1. A 0.011 mol/L solution of oxalic acid has a pH of 2.11. Calculate the Ka for oxalic acid using this information.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
HOOCCOOH(aq) + H2O(l) ![]() HOOCCOO–(aq) + H3O+(aq)
HOOCCOO–(aq) + H3O+(aq)
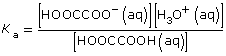
At equilibrium,
![]()
Amount Concentration |
[HOOCCOOH(aq)] |
[HOOCCOO–(aq)] |
[H3O+(aq)] |
Initial |
0.011 |
0 |
0 |
Change |
–0.0078
|
+0.0078
|
+0.0078 |
Equilibrium |
0.003 |
0.0078 |
0.0078 |
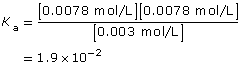
According to the data, the Ka for oxalic acid is 1.9 × 10–2.
 Read
Read
Approximations: Using Ka to Calculate the [H3O+(aq)] for an Acid
Because of their incomplete ionization, weak acids can make the calculation to estimate [H3O+(aq)] or a solution’s pH more complex than you might expect. A simple check is often done to determine whether an approximation can be used. As you will see described below, an approximation will be used if the extent of ionization for an acid is low relative to its molar concentration.
If this is the case, then the approximation can be used to reduce the complexity of the calculation required. It is important for you to understand that the reason for running the check to determine whether you can use an approximation is to predict the equilibrium concentration for the acid and to determine whether or not the predicted change in the value of the concentration is significant.
The last series of problems you looked at considered relative amounts of material involved in reactions and whether such values were significant. In “Sample problem 16.2” for example, consider the acetic acid used:
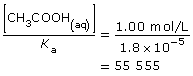
This value is higher than 1000, so the approximation is valid. As you saw in the example, subtracting the change in concentration was below the precision of the value for the initial amount concentration and was not significant.
Consider a second example, oxalic acid used in SC 1:
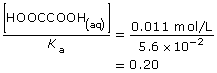
This value is significantly below 1000, so the approximation is not valid. This is consistent with the significant change in the concentration of oxalic acid due to its ionization in aqueous solution.
Are you concerned that applying an approximation will decrease the accuracy of your answer? Since the values for Ka shown on the table are only provided to two significant digits, small changes in concentration will not be accounted for in your calculations.
Remember that you must show that you understand when an approximation can be used. It is incorrect to make an assumption about the importance of the change in equilibrium concentration of the acid without showing work to back up your reasoning. The examples you have been asked to look through in the textbook demonstrate how to communicate that you are testing the assumption and how you intend to use that information to solve the problem.
 Self-Check
Self-Check
SC 2. Read the information in “Sample problem 16.3” on page 740 in the textbook. Calculate the factor difference between the concentration of methanoic acid and its Ka. Is the approximation valid for methanoic acid, or is the change in concentration during its ionization significant?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
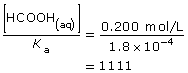
This value is higher than 1000, so the approximation is valid. As a result, the change in concentration is not significant and the initial concentration of methanoic acid, 0.200 mol/L, can be used in further calculations as required.
 Read
Read
Work through “Sample problem 16.3” and “Communication example 3” on pages 740–742 of the textbook.
 Self-Check
Self-Check
SC 3. Predict the [H3O+(aq)] and the pH for a 0.125-mol/L solution of hydrosulfuric acid.
SC 4. Complete “Practice” questions 5 and 6 on page 743 of the textbook.
 Try This
Try This
Acid-Base Reactions
It is fun to try out the concepts you are learning, and new concepts always seem to make more sense when you try them hands-on. In this simulation you will learn about percent reaction as you try reacting different acids and bases.
![]() Go to the website for your textbook. You will need your username and password, available from your teacher, to access the website. Navigate to Unit 8, Chapter 16, Section 16.3, Extensions. Once there, view the simulation “Acid-Base Reactions.” As you work with the simulation, look for patterns as you choose various reactants. Consider the following questions:
Go to the website for your textbook. You will need your username and password, available from your teacher, to access the website. Navigate to Unit 8, Chapter 16, Section 16.3, Extensions. Once there, view the simulation “Acid-Base Reactions.” As you work with the simulation, look for patterns as you choose various reactants. Consider the following questions:
TR 2. Does the Ka help you predict the extent of the reaction between the acid and a base?
TR 3. What does the position on the table indicate about the extent of the reaction?
TR 4. Explain how you could use the simulation to collect information to answer these questions.
Save your answers in your course folder.
1.25. Page 4
Module 8—Acid-Base Equilibrium
 Read
Read
Equilibrium Constant for Bases, Kb
The analysis of the equilibrium of bases has many similarities to the analyses you have completed so far in this lesson. Read from “Calculating Kb from Amount Concentrations” on page 745 in the textbook to the start of “Calculating [OH–(aq)] from Kb” on page 746.
 Self-Check
Self-Check
SC 5. Complete “Practice” questions 10–13 on page 746 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 5.
Practice 10.
Empirical Property |
Expected Result with a Strong Base |
Expected Result with a |
conductivity |
strong electrolyte |
weak electrolyte |
pH |
high |
lower, but above 7 |
rate of reaction |
higher |
lower |
Practice 11.
- CN–(aq) + H2O(l)
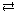 HCN(aq) + OH–(aq)
HCN(aq) + OH–(aq)
- SO42–(aq) + H2O(l)
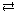 HSO4– (aq) + OH–(aq)
HSO4– (aq) + OH–(aq)
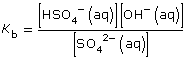
Practice 12.
| Amount Concentration | [C2H5COO–(aq)] |
[C2H5COOH(aq)] |
[OH–(aq)] |
Initial |
0.157 |
0 |
0 |
Change |
–1.1 × 10–5
|
+1.1 × 10–5
|
+1.1 × 10–5 |
Equilibrium |
0.157 |
1.1 × 10–5 |
1.1 × 10–5 |
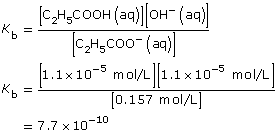
Practice 13.
![]()
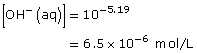
Amount Concentration |
[C6H5NH2(aq)] |
[C6H5NH3(aq)] |
[OH–(aq)] |
Initial |
0.10 |
0 |
0 |
Change |
–6.5 × 10–6
|
+6.5 × 10–6
|
+6.5 × 10–6 |
Equilibrium |
0.10 |
6.5 × 10–6 |
6.5 × 10–6 |
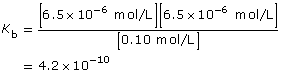
 Read
Read
A relationship exists between the three equilibrium constants you used in this module. Read pages 746–750 in the textbook, and work through the “Sample problems” and “Communication examples” in this section to learn more about the relationship between Ka, Kb, and Kw.
 Self-Check
Self-Check
SC 6. Complete “Section 16.3” questions 1 and 2 on page 750 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 6.
Section 16.3 1.
Cod(aq) + H2O(l) ![]() Cod+(aq) + OH–(aq)
Cod+(aq) + OH–(aq)
Approximation
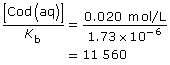
This is significantly higher than 1000; so the assumption holds, and the change in concentration in codeine is insignificant.
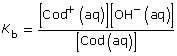
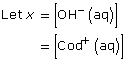
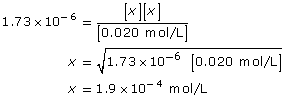
[OH–(aq)] = 1.9 × 10–4 mol/L
![]()
![]()
Section 16.3 2.
CN–(aq) + H2O(l) ![]() HCN(aq) + OH–(aq)
HCN(aq) + OH–(aq)
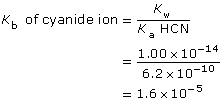
Approximation
![]()
This is greater than 1000, so the change in concentration of CN– is not significant.
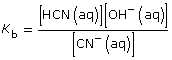
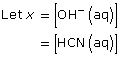
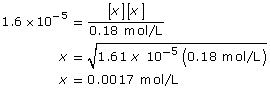
[OH–(aq)] = 0.0017 mol/L
![]()
![]()
1.26. Page 5
Module 8—Acid-Base Equilibrium
 Reflect and Connect
Reflect and Connect
In Lesson 1 you started summaries of your previous learning about acids and bases. In this lesson you investigated strong and weak acids and bases using the equilibrium law, and you learned some techniques required to complete pH and pOH calculations for weak acids and bases. You may wish to update your summaries to include concepts you have learned in this lesson.
Save a copy of your updated summaries in your course folder. You may be asked to submit a copy to your teacher.
 Module 8: Lesson 5 Assignment
Module 8: Lesson 5 Assignment
There is no assignment for this lesson.
1.27. Page 6
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following questions:
- What are Ka, Kb, and Kw?
- How do Ka and Kb explain the position of the equilibrium of aqueous acids and bases?
- How are values for Ka and Kb used to calculate the pH of solutions containing weak acids and bases?
In this lesson you used your knowledge of equilibrium systems and the ionization constants for acids (Ka), bases (Kb), and water (Kw) to analyze aqueous systems containing either acids or bases. You also used ICE tables and, where applicable, approximations to calculate equilibrium concentrations for weak acids and bases. These calculations enabled to you predict the pH of solutions containing weak acids and bases.
In the next lesson you will continue to investigate the equilibrium relationships of weak acids and bases as you investigate pH curves.
1.28. Lesson 6
Module 8—Acid-Base Equilibrium
Lesson 6—pH Curves

 Get Focused
Get Focused
In Chemistry 20 you completed a titration experiment that involved the stepwise addition of one reactant to another until an endpoint was reached. Another way to set up this experiment would have been to record the change in pH as titrant was added to the test solution. The graph of the data is a pH curve.
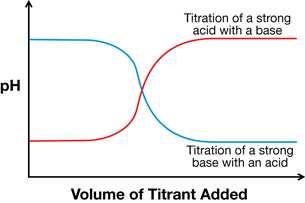
In this lesson you will revisit this graph and learn more about its unique shape, including how the shape is influenced by equilibrium. You will also learn about acidic and basic substances that can react multiple times and how their equilibrium influences the shape of a pH curve.
Consider the following questions as you complete Lesson 6:
- What information about acids and bases and their equilibrium is contained on a titration curve?
- What events occur in the reaction of polyprotic acids and bases?
 Module 8: Lesson 6 Assignment
Module 8: Lesson 6 Assignment
As part of your lesson assignment, you will sketch a pH curve. Download a copy of the Module 8: Lesson 6 Assignment to your computer. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.29. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
One of the summaries you started back in Lesson 1 focused on titration and titration curves. Can you list the equipment required to perform a titration? What is an indicator used for?
Retrieve the summary you prepared earlier and consider how your knowledge of chemical equilibrium and the equilibrium of acids and bases fits into what you know about titration curves and the process of titration.
 Read
Read
Read pages 751–752 in the textbook to learn how equilibrium concepts contribute to the unique shape of a titration curve. You may wish to add this new information on titration and titration curves to the summaries you initiated in Lesson 1.
Read pages 753–754 in the textbook to identify how indicators and the colour changes you used to detect the endpoint of a titration are part of an equilibrium system. As you read this section, consider how the dramatic change in the pH that occurs near the equivalence point of a titration becomes the stress that influences a shift in the equilibrium of the indicator system. This stress eventually leads to the predominance of the other form of the indicator molecule and its characteristic colour.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1 and 2 on pages 754–755 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
- The slope of the buffering region is relatively flat (slope = nearly 0).
- The buffering region is where the concentration of the reactant in the test solution is reducing as a result of being converted chemically due to the addition of titrant.
- Near the equivalence point, a rapid change in pH occurs; therefore, the slope of the graph is very large.
- Nearing the equivalence point, the chemical amount of titrant added is almost equal to the chemical amount of reactant in the test solution. The dramatic change in pH witnessed is a direct result of the chemical conversion of the reactant in the test solution and its decreased influence on the properties of the solution. Past the equivalence point, titrant is in excess and the pH change is due to the accumulation of titrant and its influence on the properties of the resulting solution.
Practice 2.
1.30. Page 3
Module 8—Acid-Base Equilibrium
 Read
Read
To this point you have investigated titrations in which one proton transfer occurs. In an earlier lesson in this module you learned that polyprotic acids and bases exist. What would the titration curve for a polyprotic acid or base look like?
Read pages 755–759 in the textbook to learn how the shape of a titration curve for a polyprotic species is influenced by the occurrence of another acid-base reaction. As your read this section, recall the method you used to predict acid-base reactions. At this time also read pages 760–762 to learn how the shape of a pH curve is influenced by the strength of the acid or base being titrated.
 Try This
Try This
Sketching Titration Curves
The distinctive features of a pH curve are as follows:
- starting pH
- appearance of the initial buffering region
- pH at equivalence point
- height of region between buffering regions
- number of equivalence points
- ending pH
Examine the pH curves included on pages 752–757. Describe how the distinctive aspects of titration curves identify the type of substance being titrated.
Save your response in your course folder and submit a copy to your teacher for feedback.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 8 and 9 on page 759 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 8.
- around pH 9
- phenolphthalein (pink endpoint), thymol blue (blue endpoint)
- CH3COOH(aq) + OH–(aq) → CH3COO–(aq) + H2O(l)
- The addition of titrant converts a small quantity of acetic acid into acetate ion, which has basic properties. As a result, there is a jump in pH. As titrant continues to be added, there is only a gradual change in the removal of acetic acid particles and appearance of acetation ions; so the pH change is less dramatic.
Practice 9.
a. and b.
Two reactions occur:
PO43–(aq) , Na+(aq) , H3O+(aq), Cl–(aq), H2O(l)
strongest base = PO43–(aq)
strongest acid = H3O+(aq)
PO43–(aq) + H3O+(aq) → HPO4–(aq) + H2O(l)
Since the first reaction was quantitative and titrant continues to be added, the possible reactants include:
HPO42–(aq) , Na+(aq) , H3O+(aq), Cl–(aq), H2O(l)
strongest base = HPO42–(aq)
strongest acid = H3O+(aq)
HPO42–(aq) + H3O+(aq) → H2PO4–(aq) + H2O(l)
The second reaction is quantitative as well.
The positions of H2PO4–(aq) and H3O+(aq) do not indicate a quantitative reaction would occur in a third reaction, although the reaction would favour the products.
Since there are two quantitative reactions, there would be two equivalence points on a pH curve.
c.
reaction 1: PO43–(aq) + H3O+(aq) → HPO42–(aq) + H2O(l)
reaction 2: HPO42–(aq) + H3O+(aq) → H2PO4–(aq) + H2O(l)
reaction 3: H2PO4–(aq) + H3O+(aq) ![]() H3PO4(aq) + H2O(l)
H3PO4(aq) + H2O(l)
1.31. Page 4
Module 8—Acid-Base Equilibrium
 Module 8: Lesson 6 Assignment
Module 8: Lesson 6 Assignment
Retrieve your copy of the Module 8: Lesson 6 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save your work in your course folder and submit a copy to your teacher.
 Reflect and Connect
Reflect and Connect
Retrieve the summary about titration and titration curves you started in Lesson 1 and referred to earlier in this lesson. You may wish to use this opportunity to add what you have learned in this lesson to your summary.
1.32. Page 5
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 6 you considered the following questions:
- What information about acids and bases and their equilibrium is contained on a titration curve?
- What events occur in the reaction of polyprotic acids and bases?
You expanded upon your understanding of pH curves you began in your previous chemistry course when you graphed the change in pH against the volume of titrant added. Your work here has helped you to read important information on a pH curve, including the following:
- whether the substance being titrated is an acid or a base
- whether the substance being titrated is a strong or weak acid or base
- whether the substance being titrated is monoprotic or polyprotic
With this type of information you are able to comment on the characteristics of the acid or base being studied. If necessary, you can also design appropriate experiments for further study of the acid or base under consideration.
1.33. Lesson 7
Module 8—Acid-Base Equilibrium
Lesson 7—Buffers

© Alistair Scott/iStockphoto
 Get Focused
Get Focused
In previous lessons you’ve been learning about acid deposition and the effect it can have. Why doesn’t the soil in Alberta appear to be affected? Is there something about the chemical composition of soil in Alberta that prevents damage from acid deposition?
While it may appear that acid is not having a negative effect, accumulation of acids can adversely affect other systems. Consider the example of tennis. Tennis is all about short bursts of energy. Tennis can be extremely tiring because there is little time to recover between efforts. When you move to return the ball, your body metabolizes energy to allow the muscles to contract. Sometimes the exercise is so intense that your muscles accumulate lactic acid, a by-product of metabolism.
You may recall from previous science courses that the human body is designed to function within a narrow pH range. How does the human body deal with lactic and other acids that may be present? In this lesson you will investigate the properties of buffers as equilibrium systems.
Consider the following questions as you complete Lesson 7:
- What is a buffer and how does a buffer exhibit the characteristics of an equilibrium system?
- What is buffering capacity?
 Module 8: Lesson 7 Assignment
Module 8: Lesson 7 Assignment
Download a copy of the Module 8: Lesson 7 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in this lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all of the questions and place those answers in your course folder.
1.34. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
 Read
Read
In Lesson 6 you labelled certain regions of a pH curve “buffering regions.” What happens in a buffering region compared to other regions in a pH curve? The flattened region of a pH curve indicates that little change in pH occurs despite the addition of titrant. Why so little change?
Read pages 763–765 in the textbook to learn what a buffer is and how this differs from the terms buffering and buffer capacity, which are also introduced in this section. You may wish to make a chart explaining the similarities and differences between the terms buffer, buffering, and buffer capacity and to save your chart in your course folder for future reference.
 Try This
Try This
How can your knowledge of equilibrium be used to understand the composition of a chemical buffer?
![]() Go to the website for your textbook. You will need your username and password, available from your teacher, to access the website. Navigate to Unit 8, Chapter 16, Section 16.4, Web Activities. Open the simulation “Preparation of Buffer Solutions.”
Go to the website for your textbook. You will need your username and password, available from your teacher, to access the website. Navigate to Unit 8, Chapter 16, Section 16.4, Web Activities. Open the simulation “Preparation of Buffer Solutions.”
Complete the questions in the simulation to identify what kinds of chemical components can be used to form a buffer.
 Self-Check
Self-Check
SC 1. Complete “Practice” question 18 on page 766 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 18.
- Hydrochloric acid is a strong acid; therefore, it is written as H3O+(aq) and Cl–(aq).
species: H2CO3(aq), HCO3–(aq), H2O(l), H3O+(aq), Cl–(aq)
strongest acid: H3O+(aq)
strongest base: HCO3–(aq)
H3O+(aq) + HCO3–(aq) → H2CO3(aq) + H2O(l)
- species: H2CO3(aq), HCO3–(aq), H2O(l), OH–(aq), Na+(aq)
strongest acid: H2CO3(aq)
strongest base: OH–(aq)
H2CO3(aq)+ OH–(aq) → HCO3–(aq) + H2O(l)
1.35. Page 3
Module 8—Acid-Base Equilibrium
 Self-Check
Self-Check
How can your knowledge of the equilibrium constant be used to estimate the pH of a buffer solution? Considering that the chemical components in a buffer include a weak acid and its conjugate base and water, would it be possible to determine the pH of a buffer?
SC 2. Use the following information to calculate the pH of a buffer composed of the following two solutions: 0.200 mol/L ethanoic acid and 0.100 mol/L sodium ethanoate.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
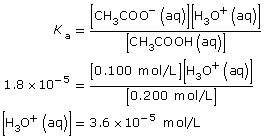
![]()
 Module 8: Lesson 7 Assignment
Module 8: Lesson 7 Assignment
Retrieve your copy of the Module 8: Lesson 7 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save your work in your course folder and submit a copy to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture
Acid deposition is a constant stress on soil. Sometimes the quantity of acids deposited overwhelms the buffering capacity of soil, and the soil pH rapidly drops.
Use the Internet to research the effect of loss of buffering capacity in soil on agricultural productivity. What strategies are used to restore the buffering capacity of soil? Do these strategies make sense in light of what you have learned in Module 8?
Save a copy of your research and your response in your course folder.
1.36. Page 4
Module 8—Acid-Base Equilibrium
 Lesson Summary
Lesson Summary
In Lesson 7 you considered the following questions:
- What is a buffer and how does a buffer exhibit the characteristics of an equilibrium system?
- What is buffering capacity?
You learned that buffers are chemical systems composed of a weak acid and its conjugate base. If these components are mixed in distinct proportions, a buffer with a specified pH can be produced.
Buffers act to maintain the pH of a system despite the addition of acid or base but, because they are composed of chemical substances with definite chemical amounts, there is a limit to the ability of a buffer to relieve a stress if excessive chemical amounts are added.
Proceed to the Module 8 Assessment, which also serves as your Unit D Assessment.
1.37. Module Summary/Assessment
Module 8—Acid-Base Equilibrium
 Module Summary
Module Summary
In Module 8 you considered the following question:
- How does the equilibrium of acids and bases affect environmental and biological systems?
You investigated the equilibrium of acids and bases. You learned to consider solutions containing acidic and basic solutes as chemical systems in which principles of equilibrium are involved. These principles include extent of reaction, equilibrium position, calculation of equilibrium constant, and determination of solution pH.
You also continued to apply your understanding of techniques to analyze equilibrium systems, including ICE tables. Throughout this module you considered environmental and biological systems and how the equilibrium of acids and bases is a part of each system’s function. This module has also provided you an opportunity to review your understanding of acids and bases developed in your previous chemistry courses and to bring together your understanding of equilibrium to broaden your base of knowledge in this area.
You also learned about buffers, and you learned how these systems demonstrate the principles of equilibrium you studied in Module 7. You examined the importance of buffers in soil and in living systems.
Concept Map or Graphic Organizer
As you worked through Module 8, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 8 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 8 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
This Assessment will serve as your Module 8 Assessment and your Unit D Assessment.
In your study in this module you have used the Relative Strengths of Acids and Bases table. Copies of this table are located on pages 8 and 9 of the Chemistry Data Booklet and on page 829 of the textbook. How is this table designed? In this Assessment you will investigate the structure and listing of information on this table. This Assessment consists of three questions.
- List, in a general way, the information that is provided on the Relative Strengths of Acids and Bases table.
- Explain how the information on the Relative Strengths of Acids and Bases table was used to support your work in Unit D. Use examples to support your response.
- Describe a series of experiments that can be used to confirm the structure and organization of the Relative Strengths of Acids and Bases table. Make sure you include the following information in your response:
- a description of experiments you would undertake
- a list of the substances to be tested
- a description of the tests to be performed and the equipment required to complete these tests
- a statement of the expected results from the experiments and tests described
- an explanation of how the expected results would confirm the organization of the Relative Strengths of Acids and Bases table
- a description of experiments you would undertake
Scoring Guide
Score |
Scoring Description |
4 |
The response is well organized and addresses all the major points of the question using appropriate and clear communication strategies. An appropriate and practical series of experiments is described. Thorough and correct justifications, interpretations, and explanations are provided. |
3
|
The response is organized and addresses most of the major points of the question using appropriate and well formed communication strategies. Appropriate and workable experiments are described. Justifications, interpretations, and explanations are correct. |
2 |
The response is generally organized and addresses most of the major points of the question using logical and reasonable communication strategies. Workable experiments are generally described. Justifications, interpretations, and explanations are correct. |
| 1 | The response is poorly organized and addresses few of the major points of the question. Poorly formed arguments and descriptions are used. A plausible experiment is suggested. Justifications, interpretations, and explanations are incorrect or absent from the response. |
| 0 | The response is not suitable for a 30-level course. |