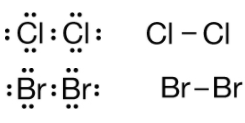Lesson 4: Chemical Bonding and Diversity of Matter
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 20 BOOTSTRAP EXAMPLE |
| Book: | Lesson 4: Chemical Bonding and Diversity of Matter |
| Printed by: | Guest user |
| Date: | Sunday, 7 December 2025, 4:42 PM |
1. Introduction
Settlers to North America used information from local First Nations people, as well as their own intuition, to develop recipes for foods, cleaning products, and weatherproofing that used natural materials. They found these substances locally or received them by trading with other groups. The substances developed by these people are examples of chemical technologies that have evolved into the commercial products used today
In the early 1900s, many scientists were asking questions regarding the bonding within chemical compounds. One of the key figures in this discussion was American chemist Gilbert Lewis. In this lesson, you will learn about Lewis’ theory and how it shaped our understanding of chemical bonding.
Key Concepts
Lewis formulas
VSEPR Theory
Molecular shapes
Intermolecular forces

2. Bonding Theory and Lewis Symbols
Electron pairing is an important concept in understanding how chemical bonds occur. Electrons occupy orbitals. Orbitals are defined as areas of space where an electron of a given energy is most likely to be found.
An orbital can hold a maximum of 2 electrons. Since electrons repel each other, electrons will occupy empty valence orbitals before pairing up.
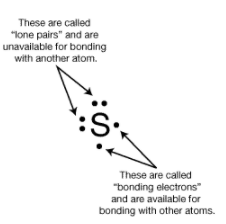
Paired electrons in an orbital are called lone pairs. Unpaired electrons in an orbital are called bonding electrons. The number of bonding electron is called the bonding capacity of the atom. Atoms with a stable bonding condition will have 2 elections in each of their 4 valence orbitals, as the situation called a stable octet or octet rule. Only the valence electrons of atoms are involved in bonding. Valence electrons are the electrons that are found in the highest energy level of atoms.
Explaining Molecular Formulas Using Lewis Symbols:
Lewis symbols ( electron-dot diagrams) are a method of keeping track of the valence electrons of atoms. In these diagrams, the element's symbol represents the nucleus and inner electrons of the atom. Each side of the symbol represents a valence orbital.
Elements in the same group of the periodic table will have a similar electron symbol because they have the same number of valence electrons. Watch the following video explaining these concepts diagrammatically.
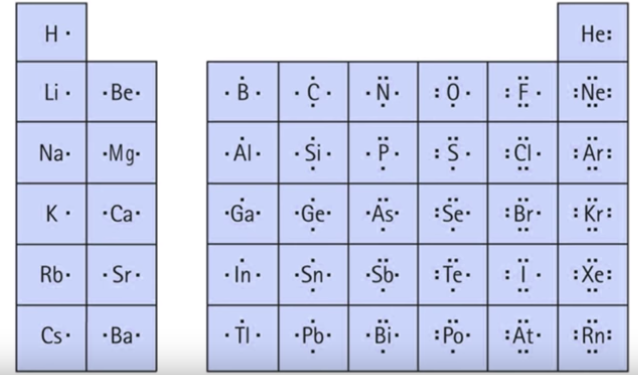
For example, the C atom has 4 valence electrons, 4 bonding, and 0 lone pair. Sulfur has 2 lone pairs and 2 bonding e-.
Molecular Elements:
There are seven elements are diatomic: Br2, I2, Cl2, H2, O2, F2. For example, Fluorine has seven valence electrons, requiring one more electron to complete its octet be become stability, meaning fill its orbitals.
![]() In the following video, I will show you how the three of these diatomic molecules are forming bonds to satisfy octet rule and duet rules in H2.
In the following video, I will show you how the three of these diatomic molecules are forming bonds to satisfy octet rule and duet rules in H2.
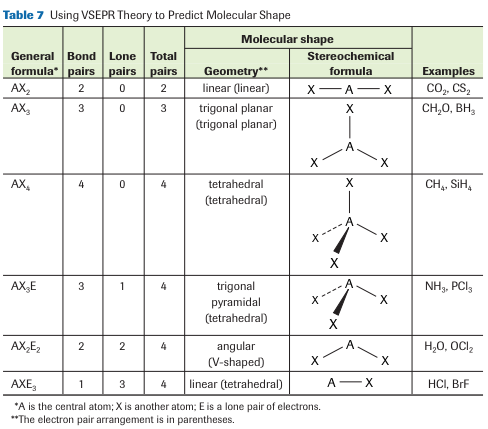
3. VSEPR Theory
Structural Diagrams
Structural diagrams are visual representations of a chemical compound that shows the relative placement of every atom with the compound. Covalent bonds are represented by the pairs of electrons shown between the Lewis symbols. Covalent bonds can also be represented using a line to represent each shared pair of electrons. For example, the structural diagram for fluorine (F2) would be written as F–F.
Valence Shell Electron Pair Repulsion (VSERP) theory is used to predict the shape of atoms surrounding the central atom of a molecule. According to VSERP theory;
- Only the valence electrons of the central atoms are important for molecular shape
- Valence electrons are paired in a molecule or polyatomic ion.
- lone pairs and bonding pairs around a central atom move as far apart as possible to minimize repulsion
- Lone-pair electrons repel with greater force than bonding-pair electrons, creating wider angles around lone electron pairs
- Multiple bonding pairs are treated as though they were single bonding pairs.
The VSEPR theory to predict molecular shapes. First, we draw Lewis formulas of the molecule, and then we rearrange all of the pairs of valence electrons to complete octets for all species involved. The key idea is that all the pairs of valence electrons repel each other and try to get as far from each other as possible. Learn more, study textbook pages 91-94.
![]() Did you know that round shape molecules are better mosquito repellent than the other shapes? Research has shown that round-shaped molecules seem better able to block the sensory nerves in the mosquito's antennae.
Did you know that round shape molecules are better mosquito repellent than the other shapes? Research has shown that round-shaped molecules seem better able to block the sensory nerves in the mosquito's antennae.
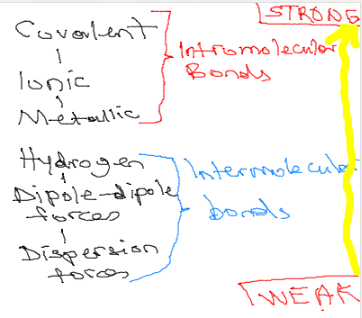
4. Intramolecular Forces
Intermolecular forces are the forces between molecules that attract one molecule to another. Without them, all substances you see around you would be in gases at all temperatures.
Van Der Waals Forces
These bonds include weak interactions between molecules. They are grouped under two major categories: London dispersion forces and dipole-dipole forces
a) London Dispersion forces are the weakest intermolecular force as they are a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms from temporary dipoles. Due to these forces that nonpolar substances condense to liquids and freeze into solids when the temperature is lowered.
The constant motion of electrons in an atom or molecule can develop instantaneous dipole when electrons distributed unsymmetrically around the nucleus.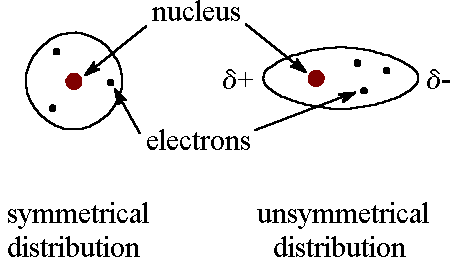
This, in turn, distorts the distribution of electrons in the second atom due to an electrostatic attraction between the + and + charges.

Dispersion forces are present between all molecules. The attraction results from this force depend on the number of electrons around the atoms involved. For example, the boiling points of the noble gases increase as one goes from He to Rn. Because larger atoms and molecules show stronger dispersion forces than smaller ones. In larger atoms, valence electrons are farther from the nuclei than in the smaller atoms or molecules. Therefore, there less tightly held and care more easily form temporary dipoles.
In non-polar molecular compounds, it is the shape of the molecule that determines the strength of the London forces. Ex. pentane (C5H12) can exist in several forms. When it is linear its boiling point is 36oC whereas it is 9.5oC when it is spherical due to the fact that linear allows for London interactions.
b) Dipole-dipole Forces: Permanent as they are, their strength depends on the electronegativity of the atoms that make up the molecule and the geometry of the molecule. These forces are due to the attraction of the positive end of one molecule to the negative end of the neighboring molecules. For example, H2O molecules have a very strong dipole because of the highly electronegative O atom compared to the H atom, and the bent shape of the molecule. CO2, on the other hand, has no dipole because the molecule is linear and the individual C-O dipoles cancel each other.
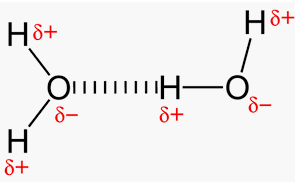
Hydrogen Bonds
Hydrogen bonds are attractive forces between a H atom covalently bonded to F, O or N in one molecule and F, O, or N of another molecule. The main factor is that F, O, and N atoms have the highest electronegativities of all the elements, so any covalent bonds between H and these elements literally leave H with a high positive charge.
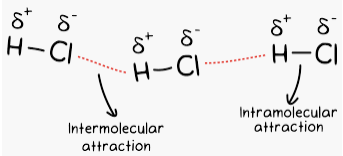
Hydrogen bonding can be intramolecular or intermolecular. Hydrogen bonding explains the high boiling point of H2O compared to those of H2S and H2Se. For example, it gives strength to various polymers (nylon, cellulose, etc) and the complementary base pairs of the DNA strands.
5. Electronegativity and Polarity
A polar covalent bond is formed when electrons between two atoms are not shared equally. The electron with higher electronegativity is partially negative, δ-, and the one with lower electronegativity is partially positive, δ+. For example, in the bond between O and H, O has a higher electronegativity and is δ-, while H is δ+. 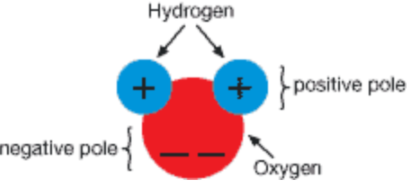
δ- ←l- δ+
O - H
3.4 - 2.2
The arrow from the diagram above represents a bond dipole, where the arrowhead is the negative end and the tail is the positive end of the polar covalent bond. It is important to note that the general trend for electronegativity is as shown in the chart below.
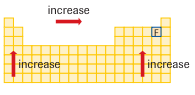
Also, study the empirical rules for polar and nonpolar molecules in the following table:
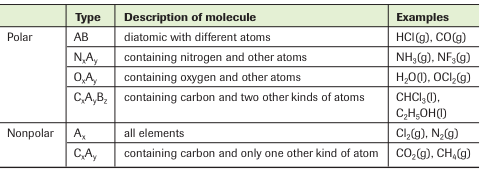
The table is certainly a great tool to determine the polarity of molecules. However, sometimes we might have to make use of other theories such as Lewis symbols and VSEPR theory to determine the polarity of molecules. Watch the video below to see how.
6. Ionic Bonding vs. Covalent Bonding
When electrons are shared, in most cases, they are not shared equally. Some atoms have a greater attraction for the shared valence electrons than the others. This relative attraction is known as electronegativity. When two H atoms have the same electronegativity and share a pair of electrons equally between them, this is called a nonpolar covalent bond.
The difference in the electronegativity of Chlorine (3.0) and Hydrogen (2.2) means that the pair of electrons in an H-Cl bond will not be shared equally. The higher electronegativity of Cl means that the electrons will stay closer to it; making the covalent bond have one end that is partially negative, and the other end partially positive. This is called a polar covalent bond.
However, sodium with an electronegativity of 0.9 and chlorine with an electronegativity of 3.0 could react. In this case, sharing would not occur at all. Instead, Na simple transfer electrons to Cl, forming Na+ and Cl- ions. The bond between Na+ and Cl- is an ionic bond.
It is important to note that there is not a sharp dividing line between ionic bonding and covalent boing. Bonding becomes more ionic and less covalent with increased electronegativity difference. So, it is safe to state that bonding between nonmetals is referred to as covalent bonding, and bonding between metals and non-metals is referred to as ionic bonding.
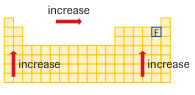 The diagram shows the electronegativity trend on the periodic table. Using the diagram above, can you identify the element with the highest and lowest electronegativity?
The diagram shows the electronegativity trend on the periodic table. Using the diagram above, can you identify the element with the highest and lowest electronegativity?