Module 5
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 5 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 10:52 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 5
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Lesson 2
- 1.8. Page 2
- 1.9. Page 3
- 1.10. Page 4
- 1.11. Page 5
- 1.12. Lesson 3
- 1.13. Page 2
- 1.14. Page 3
- 1.15. Page 4
- 1.16. Lesson 4
- 1.17. Page 2
- 1.18. Page 3
- 1.19. Page 4
- 1.20. Lesson 5
- 1.21. Page 2
- 1.22. Page 3
- 1.23. Page 4
- 1.24. Page 5
- 1.25. Page 6
- 1.26. Lesson 6
- 1.27. Page 2
- 1.28. Page 3
- 1.29. Page 4
- 1.30. Module Summary/Assessment
- 1.31. Module Glossary
1. Module 5
Module 5—Hydrocarbons and the Petroleum Industry
Module Introduction

© 2008 Jupiterimages Corporation
In Module 5 you will investigate the structure and properties of hydrocarbon molecules. You will learn about the sources of hydrocarbons, and how these natural resources are developed by society. You will learn about the industries that are involved in the extraction, refining, modification, and manipulation of hydrocarbon molecules. You will build on and apply what you learn in this module to your study of petrochemicals in Module 6.
In Module 5 you will investigate the following questions:
- What is petroleum?
- What does Alberta’s petroleum industry do?
- Where do hydrocarbons come from?
- What are hydrocarbons and what makes them such an important class of compounds?
- What are some of the processes that occur within Alberta’s petroleum industry?
- What impact does the hydrocarbon industry have on Alberta’s economy and environment?
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 5, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit C Concept Organizer. In the Module 5 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 5—Hydrocarbons and the Petroleum Industry
 Big Picture
Big Picture

© Stephen Coburn/shutterstock
As a resident of Alberta you have probably seen many pumpjacks. You might have wondered what pumpjacks are doing in the middle of fields. A pumpjack is installed into an oil well (a hole drilled deep into the ground) to mechanically lift an oil and water mixture out of the ground.
Each stroke of the pumpjack can lift between five and 30 litres of the oil and water mixture. Once the mixture is brought to the surface, the process of refining the crude oil can begin.
Crude oil is one type of fossil fuel. The number of pumpjacks you see in fields indicates that Alberta has a lot of crude oil. You may also have seen evidence of other types of fossil fuel resources being developed. For example, you may have seen evidence of coal mining and natural gas wells.
The abundance of fossil fuel resources in Alberta drives Alberta’s economy and is at the core of many industries in Alberta. The recovery and refinement of fossil fuels makes Alberta a wealthy province.
As important as fossil fuels are to Alberta’s economy, there have always been concerns about the impact the development of fossil fuel resources has on the environment. Does the extraction and refinement of fossil fuels cause irreparable damage to the environment?
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
In the Module 5 Assessment you will prepare a list of hydrocarbons. You will identify the importance of each hydrocarbon from more than just a scientific perspective.
You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 5—Hydrocarbons and the Petroleum Industry
In This Module
Lesson 1—Fossil Fuels
Have you observed evidence of the hydrocarbon industry in your local area? In Lesson 1 you will begin to investigate the kinds of activities that are involved in the extraction and processing of hydrocarbons from petroleum.
You will investigate the following lesson questions:
-
What are hydrocarbons?
-
What is petroleum?
Lesson 2—Alkanes
Once petroleum is extracted, the refining process can begin. In Lesson 2 you will learn about different kinds of hydrocarbon molecules. You will specifically learn about alkanes, a type of hydrocarbon molecule that is present in, and must be separated from, natural gas. You will also consider the use of coalbed methane as an energy source.
You will investigate the following lesson questions:
-
What are alkanes?
-
What industries use alkanes?
-
What steps are used to refine natural gas?
Lesson 3—Alkenes and Alkynes
There are hundreds of thousands of different hydrocarbon molecules. How do you communicate a particular type of hydrocarbon molecule? In Lesson 3 you will learn about the systematic naming process used to identify four more classes of hydrocarbon molecules.
You will investigate the following lesson questions:
- What are alkenes, alkynes, cycloalkenes, and cycloalkynes?
- How is the structure of an unsaturated hydrocarbon communicated?
- What is the abundance and significance of unsaturated hydrocarbons?
- How does industry use alkenes and alkynes?
Lesson 4—Aromatics
In Lesson 3 you learned that hydrocarbon molecules can exist in closed structures. One group of hydrocarbons has a unique closed structure, the aromatic ring. In Lesson 4 you will learn about aromatic compounds and how their properties make them a distinct group of hydrocarbons.
You will investigate the following lesson questions:
- What are aromatic compounds?
- How do aromatic compounds differ from other hydrocarbons?
- Why are there safety concerns associated with some aromatic compounds?
Lesson 5—Alberta’s Oil Industry
There is more to Alberta’s oil industry than pumpjacks. Alberta has heavy oil reserves and vast reserves of oil sand. In Lesson 5 you will investigate the different types of petroleum resources that are being developed in Alberta.
You will investigate the following lesson questions:
- What is crude oil?
- What is oil sand?
- How is crude oil refined?
- Why is oil sand processed?
- What products come from crude and other oil types?
- How is the oil industry affected by economics?
Lesson 6—Environmental Impact of Fossil Fuels and Alberta’s Oil Industry
In previous science courses you were introduced to the concept of a greenhouse effect. The greenhouse effect identifies a central relationship between the combustion of hydrocarbons and the warming of Earth’s atmosphere. In Lesson 6 you will review this evidence, and you will consider other local, provincial, national, and international impacts of Alberta’s hydrocarbon and petroleum industries.
You will investigate the following lesson questions:
- What processes in the petroleum industry impact the environment?
- What effect does the reaction of hydrocarbons have on the environment?
- What opinions does society have with respect to the petroleum industry?
1.3. Lesson 1
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 1—Fossil Fuels
 Get Focused
Get Focused

© José Correia Marafona/shutterstock
Are there structures like the ones shown in this picture in your community? Evidence of Alberta’s petroleum industry is part of the landscape in many parts of the province. Pipelines, flares, and refineries are obvious indications of the level of this industry’s activity in Alberta.
You may have noticed vehicles that have the names of oil and gas companies on them or the names of companies that support the exploration and extraction of oil, natural gas, and oil sand. Those are more obvious examples of the activity of the petroleum industry. Are there other examples of this industry's activity that are less obvious but that still have an impact on your community?
In Lesson 1 you will begin to explore the petroleum industry.
Consider the following questions as you complete Lesson 1:
- What are hydrocarbons?
- What is petroleum?
 Module 5: Lesson 1 Assignment
Module 5: Lesson 1 Assignment
In this lesson you will research and create a short write-up for a job in or related to the petroleum industry. Download a copy of the Module 5: Lesson 1 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all of the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
 Try This
Try This
Examine the map shown in “Figure 2” on page 359 of the textbook. Locate approximately where you live and the type(s) of fossil fuels found in your area.
Use a camera to take a picture of something that demonstrates activity around the natural petroleum resource in your area. If you do not have a camera, sketch a drawing or otherwise obtain an image of the activity. Obtain enough information to write a caption describing your photograph. Your caption should include a brief description of the object photographed, the object’s location, and any other relevant information.
Save your photograph and caption in your course folder.
![]() Post your photograph and caption to the discussion area for your class. Look at other students’ postings. Do you see any forms of activity that surprise you?
Post your photograph and caption to the discussion area for your class. Look at other students’ postings. Do you see any forms of activity that surprise you?
In your course folder, save a list of anything that surprises you or that you did not previously know about the petrochemical industry.
 Read
Read
Petroleum is a mixture of hydrocarbons. There are six main raw sources of hydrocarbons: natural gas, coalbed methane, crude oil, heavy oil, oil sands, and coal.
Read pages 356–359 in the textbook to learn more about the natural sources of hydrocarbons.
From your reading you have learned that the petroleum industry involves locating, extracting, and refining natural resources. The petrochemical industry is also part of the petroleum industry. In the petrochemical industry, chemicals created from fossil fuels are used to make products such as antifreeze windshield fluid and plastics.
In TR 1 you took a photograph as evidence of a petroleum industry activity in your area. Can you identify what aspect of the petroleum industry your photograph represents? If so, add this information to the caption you saved in your course folder.
 Self-Check
Self-Check
Complete “Case Study” questions 1–4 on page 360 and “Section 9.1” questions 1–8 on page 361 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
Case Study 1.
- Oil sands, natural gas, and conventional crude oil are the most widespread fossil fuel deposits in Alberta.
- Coal and coalbed methane are found in Alberta but are not depicted in “Figure 2.”
Case Study 2.
- Classically, research was done first and development followed later.
- Universities did the research and industry did the development.
Case Study 3.
Processing fossil fuels requires refineries for oil, upgraders for bitumen and heavy oil, processing plants for natural gas, and sorting and crushing facilities for coal. Transportation is mainly by pipelines for oil and natural gas, tanker truck for liquid fuels such as gasoline, and truck (shorter distances) and railcar (longer distances) for coal.
Case Study 4.
The following are examples of trade-offs:
- Positive outcome: Jobs are created by the tar sands industry.
- Negative consequence: When strip mining is used, it degrades the environment.
- Positive outcome: Citizens enjoy the independence and convenience of driving gasoline-powered vehicles.
Section 9.1 1.
- inorganic
- organic
- inorganic
- organic
- organic
- inorganic
- inorganic
- organic
Section 9.1 2.
Natural gas, coal, crude oil, heavy oil, oil sands, and coalbed methane are found in Alberta.
Section 9.1 3.
The two major kinds of refining are oil refining and natural gas refining.
Oil refining starts with crude oil or synthetic crude from bitumen. The crude undergoes fractional distillation followed by processing, such as hydrotreating or reforming of some of the fractions. Many products result.
In natural gas refining, the natural gas is condensed, distilled, and processed to remove impurities. The main product is methane gas. Only a few other products result, chiefly ethane, propane, butane, and pentane.
Section 9.1 4.
-
2 C4H10(g) + 13 O2(g) → 8 CO2(g) + 10 H2O(g)
2 CH3CH2CH2CH3 + 13 O=O → 8 O=C=O + 10 H-O-H
-
C5H12(l) + 8 O2(g) → 5 CO2(g) + 6 H2O(g)
CH3(CH2)3CH3 + 8 O=O → 5 O=C=O + 6 H-O-H
Section 9.1 5.
- Carbon dioxide is a greenhouse gas. As its concentration in the atmosphere builds up due to the combustion of fossil fuels, the warming effect also increases. Likely this has already affected the global climate and will affect it even more in the future.
- CO2(g) + H2O(l) → H2CO3(aq)
(carbonic acid)
Section 9.1 6.
- pro (scientific)
- pro (technological)
- pro (economic)
- con (technological)
- con (political)
- pro (legal)
- con (social, ethical)
- pro (economic)
You may have listed other perspectives on some of the statements.
Section 9.1 7.
The two major energy sources are natural gas, which is a fossil fuel, and electricity. Natural gas is used to fuel the furnace and hot water tank and, in some homes, the stove and clothes dryer too. Electricity, which powers lights and appliances, is produced mainly in power plants that burn coal or natural gas.
Your response may be a little different depending on how you interpreted the question’s use of the term energy sources.
Section 9.1 8.
Solar power, wind power, hydroelectric power, geothermal power, and biomass energy are renewable energy sources naturally available in Alberta. The question asked that you list three of these sources.
1.5. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect and Connect
Reflect and Connect
As you are approaching the end of your high school education, you may have begun to consider your career options. Many businesses and occupations in Alberta have been able to grow due to involvement either directly in or supporting the petroleum industry. Are any of the career options you are considering related to the petroleum industry?
 Module 5: Lesson 1 Assignment
Module 5: Lesson 1 Assignment
Retrieve your copy of the Module 5: Lesson 1 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment and save it in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture
Oil sands development is a hot topic these days. Alberta’s largest oil sands deposit is located around Fort McMurray. The Fort McMurray deposit is estimated to have vast hydrocarbon reserves. Development of these oil sands requires a large financial investment.
![]() View the video “The Alberta Oil Sands: A World of Opportunity” to see how the Government of Alberta is promoting the potential of the oil sands and investment in developing this natural resource. The video has been split into two parts. When Part 1 ends, view Part 2.
View the video “The Alberta Oil Sands: A World of Opportunity” to see how the Government of Alberta is promoting the potential of the oil sands and investment in developing this natural resource. The video has been split into two parts. When Part 1 ends, view Part 2.
Part 1
Part 2
 Module 5: Lesson 1 Assignment
Module 5: Lesson 1 Assignment
Submit your completed Module 5: Lesson 1 Assignment to your teacher.
1.6. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- What are hydrocarbons?
- What is petroleum?
Alberta’s petroleum industry locates, extracts, and refines natural gas, coalbed methane, crude oil, heavy oil, oil sand, and coal. In Lesson 1 you identified the type of molecules present in petroleum and some of petroleum’s chemical characteristics. You began to explore how the petroleum industry impacts your community, and you looked at some of the career options available in the petroleum industry.
Lesson Glossary
hydrocarbon: a compound containing only carbon and hydrogen atoms
petroleum: a mixture of hydrocarbons that is obtained from oil or natural gas
1.7. Lesson 2
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 2—Alkanes
 Get Focused
Get Focused

Hydrocarbons are a unique collection of molecules. Natural gas is a rich source of hydrocarbon molecules. Refineries, like the ones pictured on page 363 of the textbook and in the photo here, separate components of natural gas. In Alberta there are over 750 plants that produce and refine natural gas.
Hydrogen sulfide is a component of some natural gas resources. Hydrogen sulfide, water, and carbon dioxide are common products of natural gas refining. The final product of hydrogen sulfide removal is elemental sulfur. Judging by the size of the sulfur block pictured here, a large quantity of natural gas and hydrocarbons were refined at this facility.
In Lesson 2 you will continue to learn about hydrocarbons, and you will learn how to distinguish and communicate the differences in the chemical structure of hydrocarbon molecules.
Consider the following questions as you complete Lesson 2:
- What are alkanes?
- What industries use alkanes?
- What steps are used to refine natural gas?
 Module 5: Lesson 2 Assignment
Module 5: Lesson 2 Assignment
In this lesson you will complete a case study on the extraction of coalbed methane. Download a copy of the Module 5: Lesson 2 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.8. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
 Read
Read
Read pages 362–363 in the textbook to learn more about natural gas, including its composition and refinement.
 Self-Check
Self-Check
There are six main steps in the natural gas refining process:
-
Water and liquid hydrocarbons are removed from the gas.
-
Hydrogen sulfide gas is removed through a reaction with an amine.
-
Hydrogen sulfide gas reacts with oxygen to make sulphur dioxide.
-
The remaining hydrogen sulfide and sulphur dioxide are further recovered to make blocks of sulphur.
-
The remaining parts are cooled under high pressure to condense all components except methane.
-
The condensed liquid is distilled to further separate the components.
SC 1. Prepare a table showing which of these steps involve chemical reactions and which involve physical changes.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
| Chemical Reaction | Physical (Phase) Change |
|---|---|
steps 2, 3, 4 |
steps 1, 5, 6 |
 Read
Read
“Table 1” on page 363 of the textbook lists six hydrocarbon components of natural gas. The main components are methane, propane, ethane, and pentane. What is similar about these molecules?
Read pages 366–367 in the textbook.
The components of natural gas all end in “ane” and are considered alkanes. As hydrocarbons, alkanes are composed of only hydrogen and carbon.
Methane, ethane, propane, and butane represent the simpler alkane molecules. Regardless of the number of carbon atoms, an alkane will always have single bonds between all carbon atoms. The remaining bonds that carbon atoms can make are to hydrogen atoms.
You will recall from your work in Chemistry 20 that carbon atoms have four bonds to other atoms. For alkanes, each carbon will be chemically bonded to four other atoms, either carbon or hydrogen.
 Try This
Try This
Molecular Models of C5H12
How many different forms can a molecule with the formula C5H12 have? Use materials you find at home or at school to construct a molecular model of each of the different forms you identify. Recall the following information when constructing your representations of hydrocarbons:
- Carbon atoms can bond with up to four other atoms.
- Hydrogen atoms can only bond with one other atom.
- In alkanes, only one bond exists between two adjacent carbon atoms.
- The general chemical formula for an alkane is CnH2n + 2.
TR 1. Use a digital camera to take a picture of each of the models you constructed. If you do not have a digital camera, sketch your drawings digitally (using a program on your computer) or by hand.
TR 2. Summarize your work in a table. The table should have a column for each of the molecular models you constructed and a row for the pictures you took. Paste your pictures or sketches in your table. Later in this lesson, you will add rows to the table as you re–examine the molecules you constructed.
TR 3. Explain the principle underlying information points 1 and 2 above.
Save your work in your course folder.
 Read
Read
Methane, ethane, propane, and butane represent the simpler, linear alkane molecules. In TR 1–3 you probably noticed that the molecules you constructed had either linear appearances—all carbons connected to form a continuous chain, or branched appearances—one or more carbon atoms bonded to only a single carbon along the continuous chain.
Read pages 367–368 in the textbook to learn more about branched hydrocarbons. Complete “Sample problem 9.1” and “Communication example 1” on pages 368–369 of the textbook. In Module 6 you will learn that hydrocarbons with branches can be manufactured.
 Self-Check
Self-Check
SC 2. Retrieve a copy of the table you started in TR 2. Add a row with the heading “Systematic Name.” Use the information listed on page 368 of the textbook to write the name for each of the molecules represented in your table. Save a copy of your table in your course folder.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
|
Forms of C5H12 |
||
1 |
2 |
3 |
|
Picture |
|
|
|
Systematic Name |
pentane |
2 –methylbutane |
2,2 –dimethylpropane |
structural isomers: compounds that share the same chemical formula but have different chemical structures
alkyl branch: a group of carbon and hydrogen atoms joined by single bonds
The group is not part of the main structure of a hydrocarbon.
The different forms of C5H12 you constructed and named in TR 1–3 and SC 2 are examples of structural isomers.
The ability of a carbon atom to have up to four bonds creates the possibility of branching along the length of a hydrocarbon molecule. An alkyl branch is not part of the main chain and contains only carbon and hydrogen. Even though they have the same molecular formula, each structure is considered a different entity. Not only do the isomers have different names, but often they have differences in their properties, which can allow them to have different uses.
1.9. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Read
Read
Read “Drawing Branched Alkane Structural Formulas” and complete “Sample problem 9.2” and “Communication example 2” on pages 369–370 of the textbook.
 Self-Check
Self-Check
SC 3. Retrieve a copy of the table you last updated in SC 2. Add one row with the heading “Structural Formula,” and one row with the heading “Condensed Structural Formula.” Complete the information for the new rows of your table for each of the three isomers of pentane.
SC 4. Complete “Practice” questions 7–11 on pages 370–371 in the textbook.
 Read
Read
cycloalkanes: a cyclic (carbon chain forms a circle) hydrocarbon in which all of the bonds between carbon atoms are single bonds
Some alkanes form into cyclical structures called cycloalkanes. In these structures the carbon chain forms a circle. For more information about cycloalkanes, including how to name and draw structures for these molecules, read “Cycloalkanes” and complete “Communication example 3” and “Communication example 4” on pages 371–372 in the textbook.
 Self-Check
Self-Check
SC 5. Complete “Section 9.2” questions 1–10 on pages 372–373 of the textbook.
 Module 5: Lesson 2 Assignment
Module 5: Lesson 2 Assignment
Retrieve your copy of the Module 5: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. You will receive instructions later in this lesson on when to submit your completed Assignment to your teacher.
1.10. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect on the Big Picture
Reflect on the Big Picture

At the beginning of this lesson you saw a picture of a sulfur block, a by-product of the refining of sour natural gas. Sulfur blocks, like the one pictured, can be well over the size of a city block. Seeing a structure like that might make you think about the environmental impact of extracting, refining, and even using hydrocarbons.
Can you identify evidence of an adverse environmental impact caused by the petroleum industry in your local area? Capture any evidence you see using your camera or make a list of your observations. Save your evidence in your course folder.
 Module 5: Lesson 2 Assignment
Module 5: Lesson 2 Assignment
Submit your completed Module 5: Lesson 2 Assignment to your teacher.
1.11. Page 5
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following questions:
- What are alkanes?
- What industries use alkanes?
- What steps are used to refine natural gas?
You identified that natural gas is a source of alkanes, and that alkanes are a diverse group of hydrocarbon molecules that can have a variety of structural forms. You learned to identify the key components of alkanes, to draw their chemical structures, and to write the systematic names for an alkane and its isomers. You also began to consider the petroleum industry from a variety of perspectives, and you collected evidence of the petroleum industry's impact on your local area.
Lesson Glossary
alkanes: a class of hydrocarbons that contain only single bonds
alkyl branch: a group of carbon and hydrogen atoms joined by single bonds
The group is not part of the main structure of a hydrocarbon.
cycloalkanes: a cyclic (carbon chain forms a circle) hydrocarbon in which all of the bonds between carbon atoms are single bonds
structural isomers: compounds that share the same chemical formula but have different chemical structures
1.12. Lesson 3
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 3—Alkenes and Alkynes
 Get Focused
Get Focused

© Kiselev Andrey Valerevich/shutterstock
How careful are you about storing fruits and vegetables? Did you know that the ripening of fruits and vegetables can be influenced by other produce or even by the container in which the produce is stored? It might surprise you to know that a simple but important hydrocarbon molecule influences the ripening process.
Fruits and vegetables release ethylene, which acts as a hormone in fruits and vegetables and influences their ripening and aging. Ethylene is also called ethene and has the chemical formula C2H4.
You may notice that the name and chemical formula of ethene is different from the alkanes you studied in the previous lesson. In Lesson 1 you learned that the rules used to name hydrocarbons allowed for communicating the structure of the molecule. How might ethene be different from ethane? What aspects of these two molecules might be similar?
In Lesson 3 you will focus on other classes of hydrocarbons—alkenes, alkynes, cycloalkanes, and cycloalkenes.
Consider the following questions as you complete Lesson 3:
- What are alkenes, alkynes, cycloalkenes, and cycloalkynes?
- How is the structure of an unsaturated hydrocarbon communicated?
- What is the abundance and significance of unsaturated hydrocarbons?
- How does industry use alkenes and alkynes?
 Module 5: Lesson 3 Assignment
Module 5: Lesson 3 Assignment
The Lesson 3 assignment will assess your ability to name and write structural formulas for the types of hydrocarbons you studied in Lessons 2 and 3. Download a copy of the Module 5: Lesson 3 Assignment to your computer now. You will receive further instructions on how to complete this Assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.13. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
 Try This
Try This
Constructing Ethene
TR 1. Ethene, the hydrocarbon plant hormone, has the chemical formula C2H4. Use materials in your home or classroom to build a model of the ethene molecule. Remember that each carbon atom must bond four times in order to achieve stability.
TR 2. Write the chemical structure and the condensed chemical structure for the molecule you constructed.
Save the structure you built—you will refer back to it later in this lesson. You may wish to check your work with your teacher.
 Read
Read
Read pages 374–376 in the textbook to learn more about the structure of alkenes and alkynes and how to name and draw structures for these compounds. Complete “Communication example 1” on page 375 and “Communication example” 2 on page 376 of the textbook.
 Self-Check
Self-Check
SC 1. Use materials in your home or school to construct models or draw chemical structures for four isomers of C4H8. Complete a table like the one provided here to summarize information about the isomers of C4H8.
When building your models, remember that each carbon atom must bond four times in order to achieve stability; each hydrogen can only bond once.
|
Isomer of C4H8 |
|||
Structural Formula |
|
|
|
|
Condensed Structural Formula |
|
|
|
|
Line Diagram |
|
|
|
|
Name |
|
|
|
|
Type of Hydrocarbon |
|
|
|
|

 Read
Read
Read the section “Cycloalkenes and Cycloalkynes” on pages 376–377 in the textbook.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 1–5 and 7 on page 377 in the textbook.
 Read
Read
Alkenes and other unsaturated hydrocarbons can exist naturally but are usually found in very low quantities. As a result, they are more often made from other hydrocarbons. Because it is used to produce a variety of products, including plastics, ethane is one of the most desirable hydrocarbons.
Ethane is produced by a process called cracking. Read more about ethane cracking on pages 378–379 in the textbook.
Compare the chemical reactions for cracking shown on page 379 with the hydrogenation reaction shown on page 374 in the textbook. Can you identify any relationship, similarities, and differences between the two reaction types?
 Module 5: Lesson 3 Assignment
Module 5: Lesson 3 Assignment
Retrieve your copy of Module 5: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment and save it in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
1.14. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect and Connect
Reflect and Connect

© 2009 Jupiterimages Corporation
The fruit production industry has taken advantage of the ethene that is naturally produced by plants to better prepare fruits and vegetables for shipment, storage, and sale. By controlling the concentration of ethene around produce, the ripening process can be delayed or stimulated as required.
Inspection of produce prior to shipment is very important, because damaged fruits and vegetables produce more ethene. If damaged produce is not removed, it may be a case of one bad apple spoiling the whole bunch!
To promote ripening at home, some people place ethene-producing fruits, like bananas, peaches, pears, and nectarines, in bags with produce that is more sensitive to ethene, such as broccoli, leafy green vegetables, tomatoes, and mangos.
 Reflect on the Big Picture
Reflect on the Big Picture

In this lesson you learned that cracking reactions are used to dehydrogenate ethane extracted from natural gas. You also learned that ethene can also be produced from larger hydrocarbons extracted from other petroleum sources. In Module 6 you will learn more about reactions that use ethene, including the process used to convert ethene into plastic.
Look around the room in which you are sitting. Do you see many products made from plastic? These products are evidence of the importance of ethene and of this class of hydrocarbons.
 Module 5: Lesson 3 Assignment
Module 5: Lesson 3 Assignment
Submit your completed Module 5: Lesson 3 Assignment to your teacher.
1.15. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- What are alkenes, alkynes, cycloalkenes, and cycloalkynes?
- How is the structure of an unsaturated hydrocarbon communicated?
- What is the abundance and significance of unsaturated hydrocarbons?
- How does industry use alkenes and alkynes?
Alkenes, alkynes, cycloalkenes, and cycloalkynes are classes of hydrocarbon molecules that possess double or triple bonds. Molecules of these classes exhibit properties that make them highly useful in industrial processes.
In this lesson you were introduced to the procedures used to name and write the structures for these compounds. You also learned about some chemical reaction types that are used in their production and use. In Module 6 you will learn more about the reactivity and chemical processes involving these unsaturated hydrocarbons.
Lesson Glossary
cracking: a process in which larger hydrocarbon molecules are converted into smaller hydrocarbon molecules
1.16. Lesson 4
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 4—Aromatics
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
What happens when a gasoline station closes? Have you seen what it takes to reclaim the area? It’s pretty hard to miss the excavation of the underground fuel tank, but many of us don’t notice the removal of the soil—lots of soil!
Gasoline is a mixture of many hydrocarbons. In the past, benzene was added to fuel to improve fuel’s performance. You might have heard of benzene—researchers suspect it causes cancer.
Benzene belongs to a class of hydrocarbons, the aromatic compounds, which occur naturally but have very unique chemical properties. How do the chemical properties of benzene relate to the clean-up of land at abandoned gas stations? In Lesson 4 you will begin to learn about benzene and aromatic compounds.
Consider the following questions as you complete Lesson 4:
- What are aromatic compounds?
- How do aromatic compounds differ from other hydrocarbons?
- Why are there safety concerns associated with some aromatic compounds?
 Module 5: Lesson 4 Assignment
Module 5: Lesson 4 Assignment
There is no assignment for this lesson. However, you will be asked to submit samples of your work to your teacher where instructed.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.17. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
 Read
Read
Aromatics include benzene and all compounds that contain a benzene-like structure. The chemical formula for benzene is C6H6 but the structural diagram is more difficult to pinpoint. The structure of benzene is unique to this class of hydrocarbons. This is a unique class of compounds because of its combination of physical and chemical properties.
Read pages 381–382 in the textbook to learn more about the evidence that led to the development of a chemical structure for benzene.
 Try This
Try This
- Consider this early model for the structure of benzene.
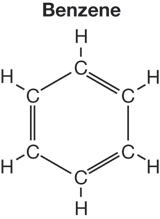
Identify one piece of evidence listed on page 381 that supports using this chemical structure as a representation for benzene and one piece of evidence that suggests this is not an appropriate representation for this molecule. Share your evidence with your teacher.
- Consider the following two illustrations:
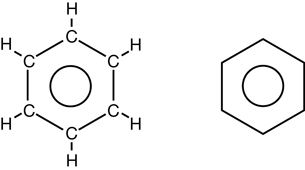
In both diagrams, the inner circle illustrates that there are not three specific double bond locations; rather, there is a way for all six carbons in the ring to bond to the remaining electrons.
 Read
Read
Read the section “Naming Aromatics” on pages 382–383 in the textbook. Complete “Communication example” and “Sample problem 9.3” on page 383.
![]() For extra reinforcement, listen to the “Communication example” audio description.
For extra reinforcement, listen to the “Communication example” audio description.
Use these strategies when naming aromatics:
- If there is an alkyl branch attached to a benzene, it is called an alkylbenzene.
- If you have more than one alkyl branch attached to a benzene, always number the benzene ring so that the numbers are the lowest possible (you will always have a branch numbered one).
- If there is a benzene ring attached to a larger molecule, the benzene is considered the branch and is called a phenyl branch.
 Self-Check
Self-Check
Complete “Section 9.4” questions 1–10 on page 385 of the textbook.
1.18. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect and Connect
Reflect and Connect
Soil contaminated with hydrocarbons can be treated using bioremediation. Bacteria that commonly live in the soil use many hydrocarbons as a food source. These bacteria remove hydrocarbons from the soil by eating them.

© EBA Engineering Consultants Limited. Used with permission.
Because of the chemical structure of the benzene ring, soil contaminated with benzene compounds is much more difficult to bioremediate. The unique bonding relationship in a benzene ring makes benzene a highly stable chemical structure. Soil and other material contaminated with benzene and other harmful aromatic compounds are often treated using special waste treatment processes.
 Reflect on the Big Picture
Reflect on the Big Picture
Benzene is currently a restricted chemical substance. Benzene and other aromatic compounds are found in crude oil and natural gas and can be separated during refining. Despite its link to cancer, benzene is used in many chemical processes.
Prepare a list of products that are produced using benzene. Research what alternatives exist to the use of benzene.
Save a copy of your list in your course folder—you might wish to refer to this list in Module 6.
 Module 5: Lesson 4 Assignment
Module 5: Lesson 4 Assignment
There is no Assignment for this lesson.
1.19. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following questions:
- What are aromatic compounds?
- How do aromatic compounds differ from other hydrocarbons?
- Why are there safety concerns associated with some aromatic compounds?
Benzene and other aromatic compounds are naturally occurring compounds that possess a similar chemical structure, the benzene ring. As you have learned in this lesson, the benzene ring is a unique chemical structure that gives this class of compounds unique chemical properties.
There are significant health concerns associated with some aromatic compounds, some of which are restricted or have to be used with appropriate caution. Some common substances, such as Aspirin (a pain reliever) and vanillin (a flavouring agent), are aromatic compounds.
Lesson Glossary
aromatics: a class of hydrocarbons in which all hydrocarbons contain benzene or a benzene-like ring
bioremediation: a process that uses natural mechanisms, including bacteria and fungi, to remove toxic materials from soil or water
1.20. Lesson 5
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 5—Alberta’s Oil Industry
 Get Focused
Get Focused

© Chris Fertnig/iStockphoto
So far in Module 5 you have learned about hydrocarbon compounds present in natural gas. Natural gas is only one of the natural sources of hydrocarbons in Alberta. As you can see in “Figure 2” on page 359 in the textbook, Alberta is also rich in hydrocarbons from oil—heavy oil, conventional crude oil, and oil sand.
Earlier in this module you were asked to look for evidence of activity in the petroleum industry in your local area. Was the activity you photographed related to natural gas, oil, or oil sand production?
In this lesson you will investigate the different types of petroleum resources that are being developed in Alberta.
Consider the following questions as you complete Lesson 5:
-
What is crude oil?
-
What is oil sand?
-
How is crude oil refined?
-
Why is oil sand processed?
-
What products come from crude and other oil types?
- How is the oil industry affected by economics?

© Rob Belknap/iStockphoto
 Module 5: Lesson 5 Assignment
Module 5: Lesson 5 Assignment
Download a copy of the Module 5: Lesson 5 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.21. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
Crude oil is a complex mixture of hydrocarbons. There are different types of crude oil. Read pages 386–387 in the textbook to learn more about the different types of crude oil, the property that determines the grade of crude oil, and the refining process.
 Try This
Try This
Refining and other processes, like dewaxing, make use of the different boiling points of molecules within oil.
![]() View the presentation “Fractional Distillation” to test your understanding of the principles and processes involved in oil refining. As you move your mouse over the possible answers in each question, a pop-up window will appear allowing you to self-score and to see where you chose incorrectly.
View the presentation “Fractional Distillation” to test your understanding of the principles and processes involved in oil refining. As you move your mouse over the possible answers in each question, a pop-up window will appear allowing you to self-score and to see where you chose incorrectly.
Only complete steps 1–10 at this time. You will complete the other steps later in this lesson.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–6 on page 388 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
Both a fractionation tower and a laboratory-scale distillation apparatus
use a heat source to boil off components of a mixture. Both
employ a vertical column to more efficiently separate and collect the fractions obtained by condensing them.
In a fractionation tower, the mixture is continuously fed in, and the fractions condense simultaneously at various levels in the tower. In the lab-scale process, a mixture is placed in the flask and the components are distilled and condensed one at a time.
Practice 2.
-
Crude oil is heated in the absence of air in the fractionation tower because, at such a high temperature, the oxygen in the air would cause the crude oil to burn.
-
C5H12(l) + heat → C5H12(g)
C8H18(l) + heat → C8H18(g)
-
C5H12(g) → C5H12(l) + heat
C8H18(g) → C8H18(l) + heat
-
Octane has a higher boiling point than pentane because octane's London forces are stronger, mainly due to octane's greater number of electrons (66 e- versus 42 e-).
-
Pentane rises higher in the tower than octane.
Practice 3.
The fractions used for cracking stock are kerosene, fuel oil, gas oil, and greases. Cracking changes products that are in less demand into products that are in greater demand. Cracking also results in products that give a greater monetary return to the refinery.
Practice 4.
Approximately 95% of petroleum is used as fuel. The major fuel produced is gasoline.
Practice 5.
-
Straight-run gasoline contains hydrocarbons with five to 12 carbon atoms, which is more than the lighter fractions but less than the heavier fractions.
-
C5H12(g) + 8 O2(g) → 5 CO2(g) + 6 H2O(g)
2 C8H18(g) + 25 O2(g) → 16 CO2(g) + 18 H2O(g)
It is assumed the fuel is vapourized before burning in the cylinder.
Practice 6.
-
The hydrocarbons are all similar nonpolar molecules, so they are mutually soluble.
-
Water molecules are very unlike hydrocarbons in that water is a highly polar hydrogen-bonded substance. There is little tendency for the crude oil solution to dissolve any water.
 Read
Read
Earlier in this module you learned that cracking is the process by which larger hydrocarbons are broken into smaller hydrocarbons. Cracking is one of the main processes used to modify molecules in crude oil into more desirable forms.
As you learned in the previous section, 95% of crude oil is used to produce fuels for heating or transportation. These fuels are a mixture of many hydrocarbons ranging in size from five to 12 carbons each. Read pages 389–391 in the textbook to learn more about the chemical processes and different types of cracking involved in refining oil.
 Try This
Try This
![]() Open the presentation “Fractional Distillation” again. Use steps 11–15 to test your understanding of chemical processes in cracking and reforming, and of alkylation used in refining oils. Select the “i” shown on each page to view simulations of the processes described.
Open the presentation “Fractional Distillation” again. Use steps 11–15 to test your understanding of chemical processes in cracking and reforming, and of alkylation used in refining oils. Select the “i” shown on each page to view simulations of the processes described.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 8–15 on pages 391–392 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 8.
If only physical means were used to refine oil, there would be too much of the heavier fractions produced, and the supply of products such as gas oil and greases would exceed the demand. Also, straight-run gasoline is of lower quality than gasoline containing compounds produced by reforming and alkylation.
Practice 9.
- CH3-(CH2)16-CH3 → CH3-(CH2)6-CH3 + CH3-(CH2)7-CH3 + C
- CH3-(CH2)8-CH3 → CH3-(CH2)3-CH3 + CH3-(CH2)2-CH3 + C
- CH3-(CH2)14-CH3 → CH3-(CH2)7-CH3 + CH3-(CH2)4-CH3 + C
Practice 10.
Hydrocracking is carried out on heavier fractions, whereas catalytic reforming is done on lighter fractions. The processes complement each other because both improve the quality of the gasoline produced at the refinery.
Practice 11.
Alkylation increases the amount of branching within the molecules. Catalytic reforming provides an increase in the amount of aromatic molecules in the gasoline.
Practice 12.
- CH3-(CH2)4-CH3 + H2 → CH3-CH3 + CH3-CH2- CH2-CH3
- CH3-CH(CH3)-CH2-CH2-CH3 + H2 → CH3-CH2-CH3 + CH3-CH2-CH3
- CH3-C(CH3)2-CH2-CH3 + H2 → CH3-CH3 + CH3-CH(CH3)-CH3
Practice 13.
- propane + pentane → 2,2,4-trimethylpentane + hydrogen
- CH3-(CH2)6-CH3 → CH3(C6H4)CH3 + 4 H2
- pentane → ethylcyclopropane + hydrogen
You may have discovered that it is not obvious that C3H5(C2H5) is a cyclic compound.
- CH3-(CH2)8-CH3 → CH3-CH(CH3)-CH(C2H5)-CH2-CH2-CH2-CH3
Practice 14.
- Benzene has the molecular formula C6H6, which gives an H:C ratio of 1:1. Even with alkyl groups attached, the ratio remains close to 1:1.
- Alkanes, alkenes, and large alkynes have a ratio close to 2:1.
- In hydrocracking, as aromatics are converted to aliphatic compounds, hydrogen is added but no carbon is removed. As a result, the H:C ratio must increase. For aromatics, the ratio increases from approximately 1:1 to approximately 2:1.
Practice 15.
Hydrocracking produces a product with a higher H:C ratio than catalytic cracking and does not produce coke (carbon), an undesirable by-product.
 Read
Read
Oil sand is a natural resource that is receiving a great deal of attention. Read page 395 in the textbook to learn more about oil sand and the extraction of the hydrocarbons, called bitumen, from oil sand.
1.22. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Module 5: Lesson 5 Lab—Factors Influencing the Extraction of Bitumen from Oil Sand
Module 5: Lesson 5 Lab—Factors Influencing the Extraction of Bitumen from Oil Sand
Background
In your reading you learned that the inventor of a process used to extract bitumen from oil sand, Karl Clark, used carefully constructed experimentation to develop his process. Experiments to improve the recovery of bitumen from oil sand are still being carried out.
Purpose
To test the effect of variables used in the extraction of bitumen from oil sand. This investigation considers the following four variables and effect hypotheses:
- temperature: The amount of bitumen extracted from oil sand increases as the temperature increases.
- pH:
The amount of bitumen extracted from oil sand increases as the pH increases.
- air: If air is bubbled into a bitumen mixture and the air bubbles are captured within the bitumen, then more bitumen floats to the top as froth.
- calcium ion concentration: Increasing the calcium ion concentration increases the quantity of bitumen froth but decreases the quality (purity) of the froth.
![]() View the virtual investigation “Factors Influencing the Extraction of Bitumen from Oil Sand.” Contact your teacher for instructions about which parts of the virtual investigation to view and how to proceed with your analysis. Remember to record data and observations as you view the presentation.
View the virtual investigation “Factors Influencing the Extraction of Bitumen from Oil Sand.” Contact your teacher for instructions about which parts of the virtual investigation to view and how to proceed with your analysis. Remember to record data and observations as you view the presentation.
 Try This
Try This
TR 1. Identify the best conditions for the extraction of bitumen from oil sand. Explain how the results from the investigation you viewed support your conclusion.
TR 2. Explain how the results of the experiments in the investigation you viewed could be useful for developing technology that improves bitumen extraction.
TR 3. Can you identify any limitations to the conclusions you could draw from these experiments? How could these limitations be addressed?
Save your responses in your course folder, and submit a copy to your teacher.
1.23. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Read
Read
Oil sand is a mixture of hydrocarbons with a different chemical composition than crude oil. Because of oil sand’s special composition, it must be refined differently than other sources of hydrocarbons, such as crude oil and natural gas.
Read page 396 of the textbook to learn more about how oil sand is refined and about how the hydrocarbons in bitumen undergo so many changes in the refining process that the end result is termed “synthetic crude oil.”
 Self-Check
Self-Check
Bitumen extracted from oil sand is a mixture containing a large proportion of aromatic hydrocarbons, whereas crude oils contain a larger proportion of aliphatic hydrocarbons. Both bitumen and crude oil are used to produce naphtha, a mixture of hydrocarbons composed of between five to 12 carbons.
SC 3. Draw chemical structures and provide names for three molecules that could be found in the naphtha fractions from the refining of bitumen and crude oil.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Sample Solution
Responses may include any aliphatic or aromatic hydrocarbons of between five to 12 carbons each. Alkanes, alkenes, cycloalkanes, cycloalkenes, and aromatic compounds are all possible answers. To check the accuracy of the chemical structures you have drawn, you may wish to check the chemical structures shown in the textbook.
1.24. Page 5
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect on the Big Picture
Reflect on the Big Picture
Crude oil refining is a billion-dollar industry. As you have learned in this lesson, Alberta is a major producer of crude oil and synthetic crude oil. However, only a small percentage of this oil is refined in Alberta. It is cheaper to transport crude through pipelines to other major centres for refining than it is to refine the oil in Alberta. The refined oil can then be shipped shorter distances to users.
View the pipeline map of North America. Can you identify the markets for Alberta oil using the map?
 Discuss
Discuss
What are the responsibilities and consequences of being a crude oil producer and exporter? Is it appropriate to export oil? These are hot topics among Albertans and others. Many people believe that the synthetic crude oil sold by Alberta is “dirty oil” because of the environmental damage caused by extracting and refining bitumen.
Write a couple of paragraphs to share your opinion on whether Alberta should extract and refine crude oil. Post your response in the discussion area for your class and read the opinions of at least two other students.
Save your work in your course folder.
 Module 5: Lesson 5 Assignment
Module 5: Lesson 5 Assignment
Retrieve your copy of the Module 5: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save your completed Assignment in your course folder, and submit a copy to your teacher.
1.25. Page 6
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following questions:
- What is crude oil?
- What is oil sand?
- How is crude oil refined?
- Why is oil sand processed?
- What products come from crude and other oil types?
- How is the oil industry affected by economics?
You learned about conventional and synthetic crude oil and its sources, and you learned about the chemical and physical processes used to refine oil.
The crude oil industry produces gasoline, diesel, liquid petroleum gases, kerosene, waxes, tars, lubricating oils, and asphalt. As you will see in Module 6, other industries use different fractions from crude oil to create a wide range of products from plastics to cosmetics.
1.26. Lesson 6
Module 5—Hydrocarbons and the Petroleum Industry
Lesson 6—Environmental Impact of Fossil Fuels and Alberta’s Oil Industry
 Get Focused
Get Focused

Far left: © JohnnyZ/shutterstock; balance: © 2008 Jupiterimages Corporation
Earlier in this module you collected photographic evidence of the petroleum industry’s activity in your local area. You may also have taken pictures of the industry's effect on your community. Even if you did not take these photographs, you can probably list many examples of the industry's impact.
In this lesson you will expand your consideration of the petroleum industry's impact. You will use the evidence you collected, including your photographs and lists, to consider the impact of hydrocarbons from fossil fuels from a local, provincial, national, and international level. You will also research current trends in, and concerns regarding, the petroleum industry and some possible directions for its future.
Consider the following questions as you complete Lesson 6:
-
What processes in the petroleum industry impact the environment?
-
What effect does the reaction of hydrocarbons have on the environment?
-
What opinions does society have with respect to the petroleum industry?
 Module 5: Lesson 6 Assignment
Module 5: Lesson 6 Assignment
Download a copy of the Module 5: Lesson 6 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
In this lesson you will also consider what you have learned about the petroleum industry, and you will identify questions about the future of this industry you feel need to be considered.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.27. Page 2
Module 5—Hydrocarbons and the Petroleum Industry
 Explore
Explore
 Read
Read

© Stephen Finn/shutterstock
The petroleum industry supplies fuel for automobiles. As a result, the petroleum and automobile industries are linked. One of the most common combustion reactions that occurs around you happens inside a car engine. From previous science courses you know there are products of combustion reactions. You may recall that some of these products can have global effects.
Cars are often lined up at drive-thrus. Their engines are running, but they aren’t going anywhere. Signs like the one shown in the photograph indicate there is concern about idling. Why the concern?
You may remember that a combustion reaction is the rapid reaction of a chemical with oxygen to produce oxides and heat. Read pages 398–400 in the textbook to learn more about combustion reactions and the concerns about tailpipe emissions and other exhaust.
 Self-Check
Self-Check
SC 1. Write two balanced equations: one for the complete and one for the incomplete combustion of pentane. Compare the oxygen needed in each equation.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
complete combustion: C5H12(l) + 8 O2(g) → 5 CO2(g) + 6 H2O(g)
incomplete combustion: 2 C5H12(l) + 11 O2(g) → 10 CO(g) + 12 H2O(g)
(one of many possible equations)
In the first equation 8 mol of O2 are required per mol of pentane. In the second equation 5.5 mol of O2 are required per mol of pentane. Complete combustion generally requires more oxygen than incomplete combustion.
Other Problems with Combustion Reactions
From your reading you know that the quantity of oxygen available to a hydrocarbon is important in ensuring complete combustion. But is ensuring complete combustion the answer to environmental concerns? In Chemistry 20 you learned about carbon dioxide, its action as a greenhouse gas, and how this has contributed to global climate change.
The quantity of uncombusted hydrocarbons present in automobile exhaust is another reason vehicle idling is a problem. These compounds can range from components initially in the fuel that do not react with oxygen at all, to new hydrocarbons produced as side products. Some of these hydrocarbons are the same compounds that are present in tobacco and other sources of smoke. You may know from health warnings that some of these compounds can be carcinogenic.
 Self-Check
Self-Check
SC 2. Use the Internet to compile two lists of hydrocarbons: one for those present in car exhaust, and one for those present in diesel exhaust. Comment on the composition of each list. Can you identify any similarities between the substances appearing in each list?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2. Many hydrocarbons are present in exhaust from both fuel types, but the percentage of each compound can be quite low. Depending upon the composition of the fuel in the research collected, benzene and toluene are present at relatively high levels. You may have identified the following trends:
- Diesel produces a greater percentage of black carbon (soot) and polycyclic aromatic hydrocarbons (PAH) than gasoline.
- Gasoline tends to produce a higher quantity of smaller hydrocarbons, which is not surprising considering gasoline is composed of hydrocarbons from a fraction of refined oil that has fewer carbons that diesel fuel.
 Read
Read
Combustion processes using fossil fuels have other unwanted side products. Nitrogen monoxide, NO(g), is a by-product of combustion reactions that occur at high temperatures, such as the temperature in an engine or furnace. Nitrogen monoxide and nitrogen dioxide are produced when NO reacts with oxygen in the atmosphere. These two compounds contribute to the production of acid deposition.
A gasoline component that contributes to the formation of acids is sulfur, often present within organic compounds in fuel. As you saw earlier, sulfur can be removed from natural gas. Efforts are underway to improve sulfur removal from other petroleum sources, including crude oil and natural gas.
Read page 393 in the textbook to learn more about the process used to remove sulfur from gasoline.
 Self-Check
Self-Check
SC 3. Identify a possible negative side effect of improved sulfur removal practices during the refining of gasoline.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3. Elemental sulfur is a product of the treatment process. Because of the large volume of gasoline produced, a large quantity of elemental sulfur is also produced. The sulfur is often stored outdoors in large blocks, which become extremely large over time.
As shown in the picture, because of their exposure to wind, rain, and snow, blocks can erode over time. The picture also shows a plastic-lined trench designed to collect water that drains from the block. The plastic lining prevents the water from seeping into the underlying soil. If it is not contained, water in this trench could become acidified and could pose a threat to biological systems.

1.28. Page 3
Module 5—Hydrocarbons and the Petroleum Industry
 Reflect on the Big Picture
Reflect on the Big Picture
Throughout your study in this module you have collected evidence of the petroleum industry's activity in your local area. You may have collected photographs, newspaper articles, or lists of events you have observed.
Go through the evidence you collected. Identify and consider some of the issues with which the evidence is connected. Use the steps below to guide your investigation.
Purpose
To consider the impact of the petroleum industry at local, provincial, and international levels.
Procedure
Step 1: Review the pieces of evidence you collected in this module. Prepare a list that identifies characteristics of the industry. For each characteristic, identify the perspectives represented. See page 45 of the textbook if you need help identifying perspectives.
For example, you may have a photograph of an equipment yard for an oilfield company. You might list a technological perspective—the machinery used to extract oil that is used to make gasoline, and an economic perspective—the company provides jobs for the local economy and taxes that support the community’s growth and other programs.
Step 2: Consider the information and perspectives from the list you prepared in Step 1. Identify one pressing issue that emerges or jumps out from your list. Describe how this issue relates to the petroleum industry.
Step 3: Can the pressing issue you identified be rephrased as a research question? For example, if your issue involved a concern about soil contamination at oil well sites, a possible research question might be, “Are there guidelines for reclaiming soil at oil well sites?”
Step 4: Type your research question into an Internet search engine. Save a copy of the search results.
Step 5: Comment on the first 15 search results. Is this an emotional issue, an issue of much debate, a focus of research, or a target for development of government policy?
Step 6: Predict how this issue will be resolved in the future. Support your prediction using evidence from your research or from other sources.
Save your work in your course folder. Contact your teacher to find out which parts of your work you should submit.
 Module 5: Lesson 6 Assignment
Module 5: Lesson 6 Assignment
Retrieve your copy of the Module 5: Lesson 6 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save your work in your course folder, and submit a copy to your teacher. Remember to ask your teacher which parts of your work from the Reflect on the Big Picture activity should also be submitted.
1.29. Page 4
Module 5—Hydrocarbons and the Petroleum Industry
 Lesson Summary
Lesson Summary
In Lesson 6 you considered the following questions:
-
What processes in the petroleum industry impact the environment?
-
What effect does the reaction of hydrocarbons have on the environment?
-
What opinions does society have with respect to the petroleum industry?
The petroleum industry relies on many complex processes. The processes are not all chemical—the industry is also shaped by political, economic, technological, ecological, and societal issues and processes. The petroleum industry’s future will be determined by considering and balancing the needs of all from a variety of perspectives.
In this lesson you applied your learning to investigating issues that exist in your local area. You might wish to continue to monitor these issues or to become more involved in acting on them.
1.30. Module Summary/Assessment
Module 5—Hydrocarbons and the Petroleum Industry
 Module Summary
Module Summary
In Module 5 you considered the following module questions:
- What is petroleum?
- What does Alberta’s petroleum industry do?
- Where do hydrocarbons come from?
- What are hydrocarbons and what makes them such an important class of compounds?
- What are some of the processes that occur within Alberta’s petroleum industry?
- What impact does the hydrocarbon industry have on Alberta’s economy and environment?
You explored the structure and properties of hydrocarbon molecules, and you learned that natural gas, crude oil, and oil sands are being developed to provide the hydrocarbons used by society.
Hydrocarbons are a unique class of molecules that can exist in many forms. In this module you saw that a similar number of carbon and hydrogen atoms can have different arrangements. These arrangements are called isomers. Hydrocarbon molecules, including their isomers, are named according to the systematic naming process, which communicates the important aspects of a molecule’s structure.
The structure and arrangement of carbon atoms provide hydrocarbons with unique chemical and physical properties. The natural gas, crude oil, and oil sands refining processes rely on these unique properties and many different reaction types. The physical property of boiling point, for example, is used to refine hydrocarbons.
Concept Map or Graphic Organizer
As you worked through Module 5, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 5 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 5 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
In this module you investigated the class of molecules called hydrocarbons. In your Module Assessment you will summarize your understanding of the importance of hydrocarbons by preparing a list of five hydrocarbons and their uses. In your list, identify and describe the importance of the five hydrocarbons from a variety of perspectives. You may wish to consult page 45 of the textbook for more information on perspectives.
Use the scoring guide provided to plan your response and to revise your work before submitting it to your teacher.
Save your completed Assessment in your course folder, and submit a copy to your teacher.
Scoring Guide
| Score | Scoring Description |
of Excellence |
The response is well organized and presents a correct and comprehensive list of hydrocarbons, their uses, and a description of their importance from all perspectives. The response uses appropriate and clear communication strategies. The description of relevant scientific, technological, and/or societal concepts is explicit. Descriptions, explanations, and/or interrelationships between the concepts provided are correct and reflect a thorough understanding. |
| 3 | The response is organized and presents a correct and comprehensive list of hydrocarbons, their uses, and a description of their importance from a range of perspectives. The response uses appropriate and clear communication strategies. The description of relevant scientific, technological, and/or societal concepts is well developed. Descriptions, explanations, and/or interrelationships between the concepts provided are correct and reflect a good understanding. |
| 2 Acceptable Standard |
The response is generally organized and presents a correct and comprehensive list of hydrocarbons along with a complete list of their uses. The description of their importance focuses on a limited range of perspectives. The response uses generally clear and adequate communication strategies. The description of relevant scientific, technological, and/or societal concepts is mentioned. Descriptions, explanations, and/or interrelationships between the concepts provided are generally correct and reflect an adequate understanding. |
| 1 | The response is poorly organized and presents an incorrect or incomplete list of hydrocarbons and their uses. The description of their importance focuses on a one or two perspectives. The response uses unclear and inadequate communication strategies. The description of relevant scientific, technological, and/or societal concepts is weak or not developed sufficiently. Descriptions, explanations, and/or interrelationships between the concepts provided reflect a poor understanding. |
0 |
The response does not reflect the expectations for a 30-level course. |
1.31. Module Glossary
Module 5—Hydrocarbons and the Petroleum Industry
Module Glossary
alkanes: a class of hydrocarbons that contain only single bonds
alkyl branch: a group of carbon and hydrogen atoms joined by single bonds
The group is not part of the main structure of a hydrocarbon.
aromatics: a class of hydrocarbons in which all hydrocarbons contain benzene or a benzene-like ring
bioremediation: a process that uses natural mechanisms, including bacteria and fungi, to remove toxic materials from soil or water
cycloalkanes: a cyclic (carbon chain forms a circle) hydrocarbon in which all of the bonds between carbon atoms are single bonds
cracking: a process in which larger hydrocarbon molecules are converted into smaller hydrocarbon molecules
hydrocarbon: a compound containing only carbon and hydrogen atoms
petroleum: a mixture of hydrocarbons that is obtained from oil or natural gas
structural isomers: compounds that share the same chemical formula but have different chemical structures


