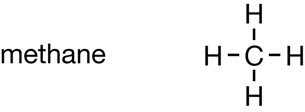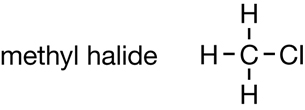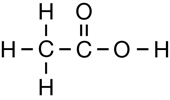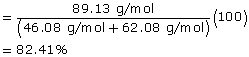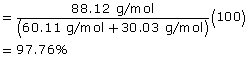Module 6
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 6 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 10:53 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 6
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Lesson 2
- 1.9. Page 2
- 1.10. Page 3
- 1.11. Page 4
- 1.12. Page 5
- 1.13. Page 6
- 1.14. Lesson 3
- 1.15. Page 2
- 1.16. Page 3
- 1.17. Page 4
- 1.18. Page 5
- 1.19. Lesson 4
- 1.20. Page 2
- 1.21. Page 3
- 1.22. Page 4
- 1.23. Page 5
- 1.24. Page 6
- 1.25. Lesson 5
- 1.26. Page 2
- 1.27. Page 3
- 1.28. Page 4
- 1.29. Lesson 6
- 1.30. Page 2
- 1.31. Page 3
- 1.32. Page 4
- 1.33. Page 5
- 1.34. Module Summary/Assessment
- 1.35. Module Glossary
1. Module 6
Module 6—Petrochemicals
Module Introduction

© jamalludin/shutterstock
In many centres in Alberta, there are areas often called “Refinery Row.” Along these roadways are many large, industrial complexes like the one pictured. In Module 5 you learned that refineries are used to process mixtures of hydrocarbons. As you will learn in this module, many of the facilities that people call refineries are not that at all—rather, they are petrochemical facilities, and they are at the core of Alberta’s petrochemical industry.
In the petrochemical industry, hydrocarbons are converted into other chemicals using a variety of chemical reaction processes. Some of these reaction types change hydrocarbons into related molecules that contain oxygen and other atoms. The resulting compounds have unique physical and chemical properties that make them useful materials for everyday products.
In Module 6 you will investigate the following questions:
-
What kinds of chemical reactions are used to manipulate hydrocarbons in order to produce petrochemicals?
-
How has the chemical industry responded to concerns about the environment?
-
What are some of the processes that occur within Alberta’s petrochemical industry?
1.1. Big Picture
Module 6—Petrochemicals
 Big Picture
Big Picture

© Joy Fera/384497/fotolia
This is a ball and stick model for the hydrocarbon molecule 2-methylpropane.
In this module you will focus on the types of chemical reactions used to convert hydrocarbons into other classes of organic compounds. These kinds of reactions are the basis of the petrochemical industry. Like most industries, the petrochemical industry is interested in “greening” its practices—making them more environmentally friendly.
As you work through this module you will investigate how the chemical industry is adapting its processes to reduce the industry’s impact on the environment. Some practices to consider when greening chemical processes are as follows:
- Prevent waste.
- Design processes that reduce or even prevent waste, have fewer by-products and more of the original material in the final product (atom economy), and use renewable materials in processes.
- Develop materials that will degrade after use by natural processes (sunlight or biodegradation)
- Reduce the toxicity of compounds.
- Design processes that involve less toxic materials, including solvents.
- Design processes so that by-products have low toxicity.
- Design processes that involve less toxic materials, including solvents.
- Improve efficiency. Efficiency can be improved in two ways:
- Strive for fewer by-products and less waste products (improved atom economy).
- Increase the use and development of catalysts to reduce energy requirements for reactions.
- Strive for fewer by-products and less waste products (improved atom economy).
- Consider safety.
- Design systems to reduce the potential for accidents and accidental exposure.
You will also learn how the green practices described are applied to chemical reactions—both the kind you will carry out in a laboratory and those that occur in petrochemical facilities.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
Aspirin (Acetylsalicylic acid, or ASA) is one of the most common drugs in the world. Aspirin can be made from compounds found in petroleum or from another starting point. At the completion of Module 6 you will use the knowledge of organic reactions you gain in this module to analyze two possible processes for manufacturing Aspirin. The Module 6 Assessment is done as part of the Unit Assessment. You may wish to look at the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 6—Petrochemicals
In This Module
Lesson 1—Petrochemicals in Alberta
In Module 5 you learned about Alberta’s petroleum reserves, but did you realize that more can be done with the petroleum? Alberta is Canada's leading petrochemical manufacturer. The petrochemical industry is Alberta’s second largest industry, and it is worth $14 billion! In Lesson 1 you will begin to explore the petrochemical industry.
You will investigate the following lesson questions:
- What are petrochemicals?
- What kinds of products result from Alberta’s petrochemical industry?
Lesson 2—Organic Halides
Among the most-used classes of petrochemicals are the organic halides. Organic halides are used in many applications, including refrigerants and in the production of plastics. In Lesson 2 you will learn how these halogen-containing molecules are made and used.
You will investigate the following lesson questions:
- What are organic halides?
- In what reactions are organic halides involved?
Lesson 3—Alcohols
You may have purchased blended gasoline recently. Gasoline can be mixed with ethanol to make blended gasoline. Ethanol is one member of a group of molecules called alcohols. Alcohols have many uses in manufacturing. As a result of their properties, alcohols are one of the most-produced petrochemicals. In Lesson 3 you will learn about the production and properties of alcohols like ethanol.
You will investigate the following lesson questions:
- What are alcohols?
- Are alcohols being produced and used in a responsible way?
Lesson 4—Carboxylic Acids and Esters
In Lesson 3 you learned about alcohols, a group of organic compounds that have a functional group containing oxygen. In Lesson 4 you will learn about two more classes of organic compounds—carboxylic acids and esters—and how the oxygen in their functional groups is critical to their chemical and physical properties.
You will investigate the following lesson questions:
- What are carboxylic acids and esters?
- How are carboxylic acids and esters involved in the petrochemical industry?
Lesson 5—Addition Polymers
Vinyl and polyethene are names you probably recognize as types of plastics. You may already know that there are many different types of plastics, each with different properties and uses. Vinyl and polyethene can only be made from certain types of petrochemcials using a specific type of reaction. In Lesson 5 you will learn about the kinds of plastics that are created using an addition reaction.
You will investigate the following lesson questions:
- What is the process used to make plastic?
- What properties must molecules have if they are to be used in making plastic?
Lesson 6—Condensation Polymers
Natural polymers include proteins, lipids, carbohydrates, and starches. In Lesson 6 you will learn how these molecules are created by polymerization reactions. You will also learn how synthetic polymers can be made using the same functional groups.
You will investigate the following lesson questions:
- How do natural and synthetic polymers compare?
- What are other reaction mechanisms by which polymers can be made?
1.3. Lesson 1
Module 6—Petrochemicals
Lesson 1—Petrochemicals in Alberta
 Get Focused
Get Focused

© Zsolt Nyulaszi/shutterstock
In the previous module you recorded signs of activity associated with the petroleum industry. You may have identified local businesses or activities in your community that use petroleum to make other products. If Alberta has a source of petroleum, it makes sense that the province should also have a lot of industry that uses petroleum.
Alberta's petrochemical industry is a very large sector of the provincial economy. It is estimated that over 7000 jobs are directly related to the petrochemical industry. You know that chemical engineers and technologists are required to operate petrochemical plants like the one pictured. However, when you consider the construction and maintenance of facilities and the support required to keep a workforce busy, you realize how many more people are indirectly employed by the petrochemical industry.
You have recently learned that petroleum involves many different kinds of molecules, but what happens in the petrochemical industry to the molecules in petroleum? In this lesson you will begin to investigate the petrochemical industry.
Consider the following questions as you complete Lesson 1:
- What are petrochemicals?
- What kinds of products result from Alberta’s petrochemical industry?
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Download a copy of the Module 6: Lesson 1 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
Later in this lesson you will also begin to record evidence and arguments from a variety of perspectives on the use of hydrocarbons as petrochemicals or as fuel. This list will be important in completing the final assessment in this module, which takes place at the end of Lesson 6.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 6—Petrochemicals
 Explore
Explore
In Module 5 you learned about hydrocarbons. The properties of hydrocarbon molecules make the molecules useful for a variety of processes. What do you know about these processes? Complete TR 1 to find out.
 Try This
Try This
TR 1. Complete “Starting Points” questions 1–3 on page 410 in the textbook. Save a copy of your answers in your course folder. Don’t worry if you don’t know the answers right now—just answer each question to the best of your ability. Later in this module you will have an opportunity to revise your answers before sharing them.
 Self-Check
Self-Check
As you get started in this module, it is important that you recall information from Module 5. You will build on that information in Module 6. Refresh your memory by completing SC 1.
SC 1. Complete “Practice” questions 1–3 on page 412 of the textbook.
 Try This
Try This
TR 2. Read through “Exploration” on page 411 in the textbook. As you work through Module 6 you will learn more about the use of molecules from petroleum. Create a document in your course folder. In this document you will record evidence and arguments from a variety of perspectives that address the resolution stated in “Exploration.” Save your document in your course folder. Later you will summarize how this issue is viewed from various perspectives.
 Read
Read
Read pages 412–413 in the textbook.
 Self-Check
Self-Check
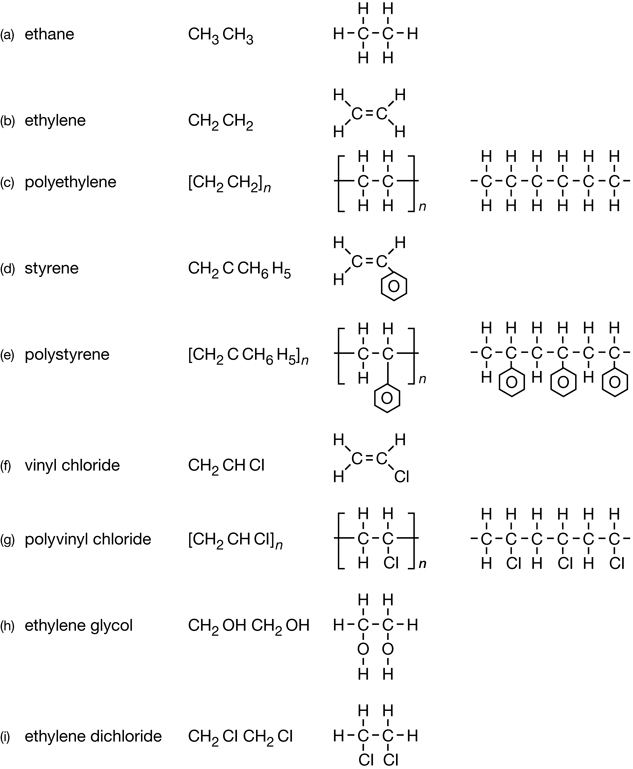
Retrieve your copy of the Module 6: Lesson 1 Assignment that you saved to your computer earlier in this lesson. Print a copy of the structural formulae that appear at the end of the document. The structural formulas are of the molecules listed in “Figure 2” on page 413 of the textbook. You may wish to use these structural formulas to answer SC 2.
SC 2. Begin your investigation of chemical reactions involved in converting petroleum into petrochemicals by answering “Practice” questions 5 and 7 on page 414 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 5.
Many answers may exist; a few examples are provided.
Practice 7.
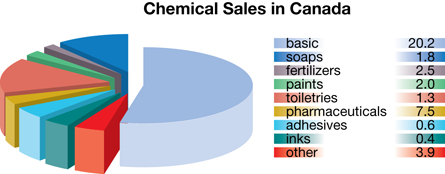
Petrochemicals

© Christos Georghiou/shutterstock
petrochemical: a chemical made or extracted from petroleum
In Alberta, the two main sources for hydrocarbons are natural gas and crude oil. Currently 95% of the natural gas and crude oil harvested is burned as an energy source. The remaining 5% is used as feedstock to create petrochemicals.
Petrochemicals include any chemicals produced from petroleum. Examples of petrochemical products include plastics, synthetic clothing, shingles, cosmetics, paint, carpets, fertilizers, adhesives, and even pharmaceuticals. The petrochemical industry is considered a secondary or tertiary industry because it takes raw materials and creates products or intermediaries from them.
Primary petrochemicals are raw chemicals that are recovered from the earth. For example, you will recall from Module 5 that ethane is a component of natural gas. It is used as feedstock to create ethene through ethane cracking. Ethene has some uses, such as ripening fruit, but most ethene is further processed to create other petrochemicals.
1.5. Page 3
Module 6—Petrochemicals
 Read
Read
Read pages 414–416 in the textbook.
 Try This
Try This
Identifying Additional Perspectives
It appears that you are living in the “Age of Oil” but, given that petroleum reserves are not infinite, there is a great deal of concern about what is being done with the resources that remain. Retrieve the document you created earlier in this lesson to record perspectives, evidence, and arguments on whether society should be saving more fossil fuels for future use as petrochemicals.
TR 3. Use the Internet to find more information on this subject. Remember to include audio and video from respected sources in your search to make sure you find documentaries and programs of interest. Try the search term “audio + CBC + age of oil” in a search engine. Review one of the websites your search returns. In your document, record the perspectives introduced and the supporting evidence and arguments. Save your work in your course folder.
 Self-Check
Self-Check
As you found in the last module, the ability to represent organic molecules as chemical structures is very important.
SC 3. Complete “Section 10.1” question 1 on page 416 of the textbook. You may wish to use molecular models when completing this question. If you are using materials in your home or classroom to build your models, remember that each carbon atom makes four bonds, each hydrogen and chlorine atom makes one bond, and each oxygen atom can make two bonds to other atoms.
SC 4. Complete “Section 10.1” question 2 on page 416 of the textbook to add to your research on the petrochemical industry and the use of petroleum. What perspective is represented in this question?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
Sc 3.
Section 10.1 1.
SC 4.
Section 10.1 2.
- For every 11 jobs in an ethene plant, 17 700 potential jobs are created.
- When ethane-containing natural gas is exported, the jobs in the ethane plant and those generated by the multiplier effect are also exported.
- The cost of ethane to plants will increase as will the cost of ethane products to consumers. The reduced availability of ethane will result in a lower supply of products made from ethane, possibly further increasing the price of the products. The high cost of these products would eventually result in a lowered demand for them and, therefore, job losses in the industry.
 Discuss
Discuss

© TebNad/shutterstock
Is society close to running out of crude oil? What will be the consequences when the crude oil supply is depleted? In this lesson you have been exposed to different perspectives on these questions.
D 1. Write a few paragraphs about your opinion on these questions, and post them to the discussion area for your class. Read the responses of at least two other students. Compare those responses to your own, and identify similarities and differences between them. Save a copy of your opinion and the similarities and differences you identified in your course folder.
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Retrieve your copy of the Module 6: Lesson 1 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment and save it in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
1.6. Page 4
Module 6—Petrochemicals
 Reflect on the Big Picture
Reflect on the Big Picture

© Dzarek/shutterstock
The plastic shopping bag is such a convenient and common item. Have you noticed recently that some stores are charging you if you want a plastic bag or are encouraging you to purchase a reusable bag made of other materials? The plastic shopping bag frequently ends up in landfills or as litter and can take thousands of years to degrade.
Some cities in Alberta are considering placing a ban or a tax on plastic shopping bags. It is hoped that this approach might significantly reduce the amount of plastic entering landfills. Plastic shopping bags like the one pictured here are made from the petrochemical polythethene. Polythethene is derived from ethane present in natural gas or is produced by cracking crude oil.
Does society’s current use of plastic bags represent an intelligent use of petrochemicals and natural resources? Does charging for, taxing, or banning the use of plastic shopping bags promote a more intelligent use of petrochemicals and petroleum?
 Discuss
Discuss
D 2. Respond to the questions listed in Reflect on the Big Picture. Post your response in the discussion area for your class. Read the responses of at least two other students in your class. Share your thoughts on the responses submitted by other students and, if necessary, revise your response. Save a copy of your response in your course folder.
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Submit your completed Module 6: Lesson 1 Assignment to your teacher.
Make sure you have updated and saved information in your document on perspectives, evidence, and arguments regarding the use of hydrocarbons as petrochemicals or as fuel.
1.7. Page 5
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- What are petrochemicals?
- What kinds of products result from Alberta’s petrochemical industry?
Hydrocarbons found in petroleum can be modified to create new substances. Approximately 5% of the natural gas and crude oil harvested in Alberta is used as feedstock to create petrochemicals. Petrochemicals include all chemicals that are produced from petroleum. Plastics, synthetic clothing, shingles, cosmetics, paint, carpets, fertilizers, adhesives, and pharmaceuticals are examples of petrochemical products.
Petrochemicals are one way to use the hydrocarbons found in petroleum. As you work through Module 6 you will continue to collect information and consider different perspectives on how society can best use the hydrocarbons found in petroleum.
Lesson Glossary
petrochemical: a chemical made or extracted from petroleum
1.8. Lesson 2
Module 6—Petrochemicals
Lesson 2—Organic Halides
 Get Focused
Get Focused

© Diego Cervo/shutterstock
Hey, teenagers are still growing, and they are often hungry. So it makes sense that the refrigerator door might be the first door you open in the morning and the last door you close at night. At one time, homes relied on an icebox and daily deliveries of ice to refrigerate food. Today, refrigeration is a heavily relied-upon technology.
Did you know that the technology used to operate a modern refrigerator relies on the energy change that occurs when certain molecules undergo a phase change? This technology goes back to the mid-1700s. Unfortunately, the first refrigerants (like diethyl ether and ammonia) posed a health risk and were not the safest substances to use.
In a quest to find safer refrigerants, a class of organic compounds called chlorofluorocarbons (CFCs) were developed and tested. The first CFCs were found to have chemical and physical properties suitable for use in refrigeration systems, and they were non-toxic. As a result, CFCs were quickly adopted for use as refrigerants.
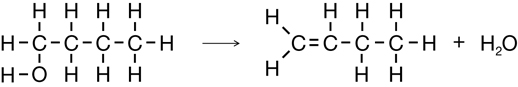
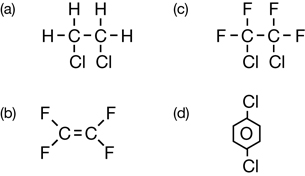
Consider the structural formulae for two CFCs:

CFCs and other organic molecules can contain halogens. In Lesson 2 you will learn about organic halides, including how they are made and used. Organic halides are halogen-containing organic molecules.
Consider the following questions as you complete Lesson 2:
- What are organic halides?
-
In what reactions are organic halides involved?
 Module 6: Lesson 2 Assignment
Module 6: Lesson 2 Assignment
There is no assignment for this lesson. However, you will be asked to submit samples of your work to your teacher where instructed.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.9. Page 2
Module 6—Petrochemicals
 Explore
Explore
hydrocarbon derivative: a molecular compound of carbon, at least one other element, and, usually, hydrogen
functional group: a characteristic arrangement of atoms or bonds within a molecule that determines the most important chemical and physical properties of a class of compounds
In Lesson 1 you examined the chemical structures of many petrochemicals, and you organized the petrochemicals into groups. Can you recall what strategy you used to group these molecules? Did your strategy involve grouping all the molecules that had halogen atoms into one group and all the structures with oxygen atoms into another group? If so, you were grouping the petrochemicals into hydrocarbon derivative families.
Atoms other than carbon or hydrogen, or the presence of multiple bonds between carbon atoms in a hydrocarbon, create a functional group. In this module you will learn about the functional groups present in common petrochemical compounds.
You may recall from “Figure 2” on page 413 that petrochemical derivatives are created by altering hydrocarbons (or primary petrochemicals). Compare the following two illustrations:
Hydrocarbon |
Petrochemical Derivative |
|
|
How could you use the hydrocarbon on the left to create the molecule on the right? Describe the changes that need to occur to make the molecule on the left transform into the one on the right.

Organic compounds that contain halogen atoms are grouped as organic halides. Organic halides contain one or more atoms from the halogen group of elements. As mentioned in Get Focused, chlorofluorocarbons are an excellent example of an organic halide because the class contains both chlorine and fluorine.
Vinyl chloride is another petrochemical example of an organic halide. As these examples show, an organic halide’s name communicates the halogen(s) present in the substance. Later in this lesson you will learn how to apply the systematic naming system to organic halides.
 Try This
Try This
Refrigeration
Halogen atoms are added to hydrocarbons to produce a compound with unique properties. Consider the following molecules:
Methane |
CFC Molecule |
|
|---|---|---|
| Chemical Formula | CH4 |
CF2Cl2 |
| Boiling Point (°C) | −161 |
−29.8 |
TR 1. Can you identify a reason for the difference in boiling points between these two molecules?
TR 2. In a refrigeration system, refrigerants are compressed into their liquid form and allowed to vapourize. Explain how the vapourization of either of the substances shown in the table would enable them to act as refrigerants.
Save your response in your course folder, and submit a copy to your teacher.
 Read
Read
Read page 417 in the textbook to learn the effect that adding halogens to a hydrocarbon can have. Pay careful attention to the last paragraph and “Table 1” on page 417 to learn how to apply the systematic naming process to organic halides.
Work through “Sample problem 10.1,” “Communication example 1,” and “Communication example 2” on page 418 in the textbook.
Earlier in this lesson you considered the CFC molecule CF2Cl2. What is the systematic name for this molecule? Is it dichlorodifluoromethane or difluorodichloromethane—which halogen do you list first?
IUPAC rules state that when more than one type of halogen atom appears in a molecule's structure, the halogen prefixes are to be listed in alphabetical order. Therefore, the systematic name for CF2Cl2 is dichlorodifluoromethane.
 Self-Check
Self-Check
Sc 1. Complete “Practice” questions 1–5 on pages 418–419 in the textbook.
Sc 2. Write the systematic names for the organic halides listed in “Practice” questions 4.b., c., and d., and 5.a., b., and d. on pages 418–419 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
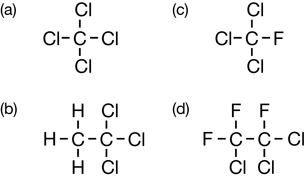
SC 1.
Practice 1.
Practice 2.
- triiodomethane
- 3-chloromethylpropene
- dichloromethane
- 1,2,3-tribromopropane
- chlorobenzene
- phenylethene
You might have used names that contain redundancies; these are still correct. For example, 3-chloro-2-methylprop-1-ene is acceptable for b. above.
Practice 3.
- Chloroethene has a higher boiling point than ethene because it has stronger intermolecular forces. Firstly, it is polar so it exhibits dipole-dipole forces. Secondly, it has more electrons so its London forces are stronger.
- Chloroethene is likely more soluble than ethane because it is polar and water is a polar solvent.
Practice 4.
Practice 5.
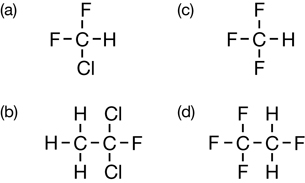
SC 2.
4.b. 1,1,1-trichloroethane
4.c. trichlorofluoromethane
4.d. 1,1,2-trichloro-1,2,2-trifluoroethane
5.a. chlorodifluoromethane
5.b. 1,1-dichloro-2-fluoroethane
5.d. 1,1,1,2-tetrafluoroethane
 Reflect and Connect
Reflect and Connect
DDT (dichlorodiphenyltrichloroethane, a pesticide), Teflon (polytetrafluoroethylene, the non-stick coating used in cookware), and PVC (polyvinyl chloride, a type of plastic) are other organic halides. Chemical technologies like these have been very important in the development of your society and to your standard of living. However, there are also concerns related to the use of organic halides.
RC 1. Use the Internet to research organic halides and possible concerns related to their use. Why is there concern about exposure to organic halides that contain chlorine (also known as organochlorides)? To refine your search, try combining the common names of the chemical compounds listed in the paragraph above with other search terms.
Save relevant information that you collect in your course folder. You will revisit this information later in Module 6.
1.10. Page 3
Module 6—Petrochemicals
 Read
Read
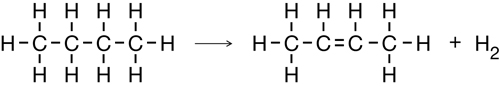
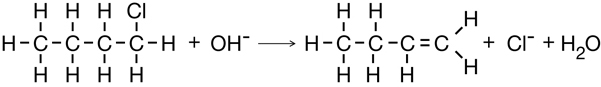
You might recall from previous science courses that chemical reactions can be classified. You will also recall that certain reaction types tend to happen with certain reactants. A multiple bond between carbon atoms, like those found in alkenes and alkynes, can participate in a type of reaction called an addition reaction.
Read pages 419–421 in the textbook to learn how addition reactions can be used to produce organic halides.
Early in this lesson you were asked to consider how you could use a hydrocarbon molecule to create a hydrocarbon derivative and to describe the changes that needed to occur to transform the molecule into the derivative.
substitution reaction: a type of organic reaction involving breaking a carbon-hydrogen bond and replacing the hydrogen atom with another atom or group of atoms
If you thought this change required substituting a hydrogen atom for a chlorine atom, you were well on your way to understanding substitution reactions, another reaction type used to make organic halides.
Read pages 421–423 in the textbook to learn more about substitution reactions.
 Self-Check
Self-Check
SC 3. Complete “Practice” questions 6–11 on page 422–423 in the textbook.
1.11. Page 4
Module 6—Petrochemicals
 Watch and Listen
Watch and Listen
What does it look like when an alkene reacts and undergoes an addition reaction? View the video “Double Bonds” to find out. As you watch the video, carefully observe changes that occur to the substances involved.
Use the results of the test performed at the end of the video to answer Try This questions 3–6.
 Try This
Try This
Addition Reactions
TR 3. Prepare a data table for the tests performed during the investigation “Double Bonds.”
TR 4. Write the balanced chemical equation for the reaction between cyclohexene and bromine.
TR 5. Which substance, corn oil or octane, contains double bonds? Use the evidence from the investigation to support your answer.
TR 6. Fats that contain double bonds are often called unsaturated fats. A common test to determine the extent of the unsaturation is to perform a titration using the halogen iodine. It is observed that a 1:1 mole ratio exists between the moles of iodine that will react to the moles of double bonds in the fat. Explain why such a relationship is observed.
Save your responses in your course folder, and submit a copy to your teacher.
1.12. Page 5
Module 6—Petrochemicals
 Reflect on the Big Picture
Reflect on the Big Picture

© Chepko Danil Vitalevich/shutterstock
The effects of a technology are often realized some time after the technology is put into use. In this lesson you completed a Reflect and Connect activity in which you began to record some issues related to the use of organic halide compounds.
You have learned that organic halides are often used as solvents, often in chemical processes. A major concern is their toxicity and lack of ability to biodegrade. To respond to some of these issues, the chemical industry is taking steps to reduce possible environmental impacts.
Green chemistry describes principles that should be considered when designing industrial processes and even when using chemicals in laboratories or at home. A list of these principles appears in the Unit C Conclusion.
Consider the following two principles of green chemistry:
- Design safer chemicals and by-products—design chemical products to have little or no toxicity.
- Design chemicals and products to degrade after use—products should be able to degrade to harmless substances and should not accumulate in the environment.
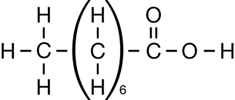
Retrieve your copy of the Module 6: Lesson 1 Assignment that you saved to your computer in Lesson 1. Look at how you organized the chemical structures for the compounds shown in “Figure 2” on page 413 of the textbook. Did you group the three organic halides together?
RBP 1. Review the diagrams of the petrochemicals from the Lesson 1 Assignment, and list the three organic halides. Use the Internet to research each of the three organic halide petrochemicals you listed. Consider the following questions:
- How is this substance used?
- Is this substance considered a toxic substance?
- Is this substance biodegradable?
Summarize the results of your research in a brief table. Save a copy of your response in your course folder.
In recent years there has been much debate about the effects of chlorine use on the environment. In Module 3 you learned that chlorine is a strong oxidizing agent, meaning that it can readily react with many substances, including organic compounds. These reactions are used to disinfect water, cause bleaching, and to make petrochemicals resulting in plastics. Many people are concerned that chlorine introduced in organic halides can become involved in other chemical change, including reactions within biological systems that could be harmful.
In another example, the burning of polyvinyl chloride (PVC plastics) can produce a number of compounds including the toxic, chlorine-containing compound, dioxin. Given such possibilities, some think that chlorine use by chemical and other industries should be banned. An Internet search will produce much more information about this debate from the scientific community and from other perspectives.
 Module 6: Lesson 2 Assignment
Module 6: Lesson 2 Assignment
There is no assignment for this lesson.
1.13. Page 6
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
It might surprise you to know that one of the foundations of Alberta’s petrochemical industry was chlorine. One of the main reasons that Dow Chemical chose to establish a petrochemical facility in Fort Saskatchewan was the location’s proximity to the underground mineral deposits containing NaCl. The chlorine produced at the Fort Saskatchewan facility was used by another plant at the same facility to produce the organic halides ethylene dichloride, vinyl chloride, and polyvinyl chloride.
In Module 3 you completed an electrolysis of aqueous sodium chloride, which produced chlorine gas as a product. You may wish to revisit the Module 3 investigation and “The Chlor-Alkali Process” on page 648 in the textbook.
In Lesson 2 you considered the following questions:
- What are organic halides?
-
In what reactions are organic halides involved?
Organic halides are an important class of organic compound and are used in a variety of important technological applications. It will not surprise you that, because of this importance, large quantities of these substances have been produced in petrochemical plants in Alberta and in other parts of the world. Addition and substitution reactions are the two reaction types currently used to produce organic halides.
Organic halides are also at the centre of some controversy. Plans to rethink how chemicals are made and used may help address concerns.
Lesson Glossary
addition reaction: a type of organic reaction involving an unsaturated hydrocarbon and a small molecule, usually hydrogen or a halogen, that increases the saturation of the hydrocarbon
chlorofluorocarbons (CFCs): a group of organic compounds composed of carbon, hydrogen, chlorine, and fluorine
functional group: a characteristic arrangement of atoms or bonds within a molecule that determines the most important chemical and physical properties of a class of compounds
hydrocarbon derivative: a molecular compound of carbon, at least one other element, and, usually, hydrogen
organic halide: an organic compound in which one or more of the hydrogen atoms has been replaced by halogen atoms
substitution reaction: a type of organic reaction involving breaking a carbon-hydrogen bond and replacing the hydrogen atom with another atom or group of atoms
1.14. Lesson 3
Module 6—Petrochemicals
Lesson 3—Alcohols
 Get Focused
Get Focused

© Artem Efimov/shutterstock
You may have heard of biofuels being used to fuel automobiles. Biofuel, or gasohol as it is called in many countries, is a mixture of gasoline and ethanol. In Canada you can purchase automobile fuel with up to ten percent ethanol. In countries like Brazil, the ethanol percentage is significantly higher.
Blending petroleum and ethanol is somewhat like diluting the petroleum component of gasoline while ensuring the energy content of the fuel. In Brazil, where all petroleum is imported but there are substantial resources to make ethanol, blending allows the country to make the greatest use of its resources.
Like other petrochemicals, ethanol can be produced from the hydrocarbon ethene using an addition reaction. Ethanol can also be produced by fermenting corn and other crops. Is producing ethanol by fermentation a more preferred option than producing ethanol by chemical synthesis?
In Lesson 3 you will learn about the different methods of producing ethanol and the properties of similar molecules called alcohols.
Consider the following questions as you complete Lesson 3:
- What are alcohols?
- Are alcohols being produced and used in a responsible way?
 Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
Download a copy of Module 6: Lesson 3 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.15. Page 2
Module 6—Petrochemicals
 Read
Read
Alcohols are another type of hydrocarbon derivative and a commonly produced petrochemical. Read pages 425–426 in the textbook to learn more about alcohols.
Two alcohols, methanol and ethanol, were discussed in the section you just read. What is the difference between these two substances? How do their names convey information about the chemical structures of each compound?
Read pages 427–431 in the textbook for more information on how alcohols are named. Work through the “Sample problems” and “Communication examples” provided on these pages to practise your skills in naming and writing chemical structures for alcohols.
 Self-Check
Self-Check
SC 1. Complete “Practice” question 4 on page 426 and “Practice” questions 5–17 on pages 430–431 of the textbook.
1.16. Page 3
Module 6—Petrochemicals
 Read
Read
Alcohols like methanol and ethanol can be reactants in chemical processes that produce other petrochemicals. Read “Elimination Reactions” on pages 431–433 in the textbook.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 18–20 on page 433 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 18.
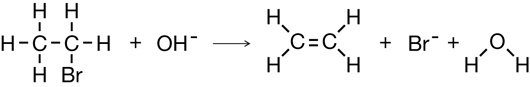
Practice 19.
Practice 20.
Cracking of Propane
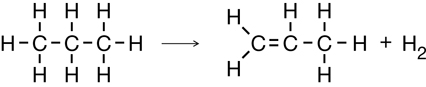
Elimination in Strongly Basic Conditions (Dehalogenation)
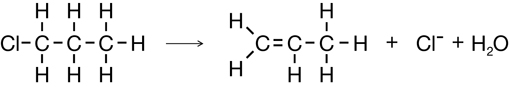
Elimination of an Alcohol
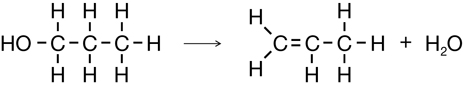
 Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
Retrieve the copy of the Module 6: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete Part 1 of the Assignment. You will receive information later in the lesson on when to complete Part 2 and on when to submit your Assignment to your teacher.
1.17. Page 4
Module 6—Petrochemicals
 Reflect and Connect
Reflect and Connect

© James Steidl/shutterstock
Ethanol is being championed around the world as a great fuel additive for blending with gasoline. One day, ethanol might even be a substitute for gasoline. Ethanol is environmentally sound because it can be made through yeast fermentation from renewable resources such as corn, sugar cane, and switch grass.
It may seem ideal to use a renewable resource to make ethanol; however, there are concerns about how much land the process requires. Some people argue that the land used to grow the plants for ethanol production could be used to grow food for the world’s hungry.
A greener option for ethanol production may be to use waste products. Research is being done to find a way to use agricultural waste (such as straw) or pulp and paper waste (such as wood chips) for ethanol production. This would resolve the land-use concern and would make use of an otherwise unused waste product.
 Try This
Try This
Cellulosic Ethanol
TR 1. Take a deeper look into the production of ethanol as a biofuel. Open “Web Activity: Web Quest—Cellulosic Ethanol.” Read the instructions and discuss which parts of the project you will be responsible for with your teacher. Complete the requirements for the activity as described in the document. Go to the website for the textbook if you need live links to the websites and documents listed. Information to help you find the activity on the website is contained in the print version linked into this paragraph.
When you have completed the activity, contact your teacher for further instructions on submitting and/or sharing your presentation. Save a copy of your work and presentation in your course folder.
 Module 6: Lesson 3 Assignment
Module 6: Lesson 3 Assignment
Retrieve the copy of the Module 6: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete Part 2 of the Assignment. To complete this part you will need to review the information collected for your presentation in TR 1. You may also be able to review the presentations of other students by using the discussion area for your class.
Submit your completed Module 6: Lesson 3 Assignment to your teacher.
1.18. Page 5
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following questions:
- What are alcohols?
- Are alcohols being produced and used in a responsible way?
Alcohols are a group of organic compounds containing a hydroxyl group. Alcohols can be produced by chemical synthesis or, as is the case in ethanol production, by plant fermentation. Alcohols are a very useful group of organic compounds due to their chemical and physical properties. For example, alcohols are often used as solvents in chemical processes, in antifreezes, and as a reactant in elimination and other reactions with organic compounds.
Your work in this lesson, and later in this module, will show you that alcohols are a very important class of organic compounds, not only because of their ease of combustion and high energy content, but because of their use in synthetic reactions. In the next lesson you will learn about esterification reactions, another type of organic reaction in which an alcohol is a reactant.
Many countries are looking into ethanol as a fuel additive or even as a fuel alternative, because ethanol is a renewable resource. The use of ethanol as a biofuel has sparked a great deal of interest and concern. In Lesson 3 you considered many of the perspectives associated with this issue.
Lesson Glossary
alcohol: an organic compound containing one or more hydroxyl groups as the functional group
1.19. Lesson 4
Module 6—Petrochemicals
Lesson 4—Carboxylic Acids and Esters
 Get Focused
Get Focused

In Unit A you learned about biodiesel, an alternative automobile fuel made from waste cooking oil. Finding a use for waste oils and other compounds that are waste products of other processes makes good sense. It is not surprising that finding a use for “waste” materials is consistent with the principles of green chemistry, since this practice does not allow for substances to accumulate without a purpose.
Examine the biodiesel reaction shown below. Do you recognize a familiar chemical structure, one that would belong to a group of molecules introduced in a previous lesson in this module?
Biodiesel Reaction
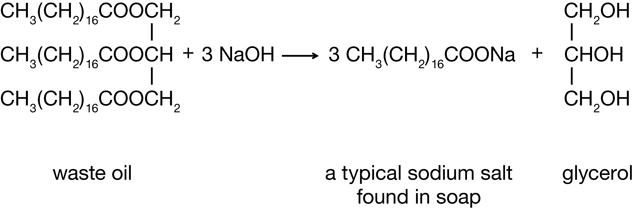
You may have noticed that an alcohol is a product of the reaction. In Lesson 3 you learned about alcohols—a type of organic compound that contains an oxygen atom in its functional group. If you look closely at the reactant and other products of the reaction, you will notice that there are two other types of organic compounds containing oxygen. These two classes of molecules will be the focus of this lesson. In Lesson 4 you will learn about carboxylic acids and esters.
Consider the following questions as you complete Lesson 4:
-
What are carboxylic acids and esters?
-
How are carboxylic acids and esters involved in the petrochemical industry?
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
Download a copy of the Module 6: Lesson 4 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.20. Page 2
Module 6—Petrochemicals
 Explore
Explore
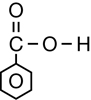
The second class of organic molecules that contains oxygen atoms is the carboxylic acids family. Read pages 436–437 in the textbook to learn more about carboxylic acids.
Carboxylic acids always contain the carboxyl functional group, which is illustrated below. Work through “Sample problem 10.5” and “Communication example 1” on page 438 in the textbook.
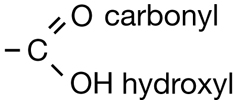
You might note that in the reaction for biodiesel shown in Get Focused, the first product—the carboxylic acid—is not complete; it is deficient a hydrogen atom. The hydrogen in question is part of the hydroxyl group, within the carboxyl group. In Unit D you will learn more about the reactions and acidity of carboxylic acids. You will also learn how a carboxylic acid can be correctly represented in the form shown in the biodiesel equation.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–2 on page 438 in the textbook to practise naming and drawing chemical structures for carboxylic acids.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
Practice 2.
- methanoic acid (you may hear the common name “formic acid”)
- pentanoic acid (you may hear the common name “valeric acid”)
- hexanoic acid (you may hear the common name “caproic acid”)
 Read
Read
esterification reaction: a chemical reaction in which a carboxylic acid and an alcohol combine to produce an ester and water
esters: a family of organic compounds characterized by the ester functional group (-COO-)
In Module 6 you have studied many kinds of organic reactions including substitution, fermentation, cracking, addition, and elimination. Another major reaction of carboxylic acids is an esterification reaction. Read pages 438–440 in the textbook to learn about this type of chemical reaction and its product—esters.
Work through “Sample problem 10.6,” “Sample problem 10.7,” and Communication example 2” on page 440 in the textbook.
 Watch and Listen
Watch and Listen
Listen to the audio clip “Naming and Preparing Esters” to better your understanding of esters. References made to “the margin” are references to the margins of the textbook.
You may wish to save a print copy of “Naming and Preparing Esters.”
 Reflect and Connect
Reflect and Connect
RC 1. Compare the two processes shown in the reactions below. Identify similarities and differences between the two reactions.
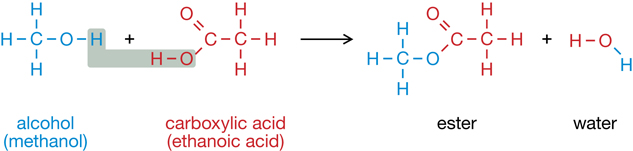

You may have noticed that these two reactions involve the same reactants and products but are each other’s reverse—one creates an ester and the other breaks down an ester.
Interestingly, esters are used in making soap. Animal fats and vegetable oils are esters and are a key ingredient in making soap. When esters are combined with a strong base, sodium hydroxide for example, and heated, the reaction creates a salt of a carboxylic acid (the soap), and glycerol (propane-1,2,3-triol).
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 4–6 on page 441 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 4.
- ethyl propanoate (from propanoic acid and ethanol)
- methyl butanoate (from butanoic acid and methanol)
- butyl methanoate (from methanoic acid and butan-1-ol)
- propyl ethanoate (from ethanoic acid and propan-1-ol)
If you got the two fragments backwards when you named the esters in this question (e.g., propyl ethanoate for part a above), try writing the full structural formula for each—it will help you get the name right.
Practice 5.
- As shown in “Table 2” on page 439 of the textbook, the ester is pentyl butanoate. The acid required is butanoic acid and the alcohol needed is pentan-1-ol.
- As shown in the same table, the ester is octyl ethanoate. The acid required is ethanoic acid and the alcohol needed is octan-1-ol.
Practice 6.
- For each class of compounds, as the molar mass increases so does the number of electrons. The increase in the number of electrons strengthens the London forces attracting the molecules to each other, thus increasing the boiling point.
- The alkanes exhibit only London forces. Even though the esters are polar and have dipole-dipole forces as well as London forces, the effect is negligible in large molecules where only the small area around the oxygen atoms is polar.
- Of the three classes, only the carboxylic acids contain the O-H bond. Because this feature gives them hydrogen bonding, these compounds have stronger intermolecular bonding and, therefore, higher boiling points.
- Yes. Knowledge of London forces, dipole-dipole interactions, and hydrogen bonding explain the observed properties.
- The effect of molecular shape on London forces must be considered in branched-chain structures.
- The line for alcohols would fall between those of methyl esters and carboxylic acids.
- The prediction in part f is verified. (If you made the wrong prediction, check that you remembered that alcohols have H-bonding.)
1.21. Page 3
Module 6—Petrochemicals
 Module 6: Lesson 4 Lab—Synthesizing Esters
Module 6: Lesson 4 Lab—Synthesizing Esters
Purpose
You will prepare a synthetic, organic compound—an ester.
![]() Retrieve your copy of the Module 6: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Then, open the handout “Making Esters.” Complete the Pre-Lab question on the Assignment.
Retrieve your copy of the Module 6: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Then, open the handout “Making Esters.” Complete the Pre-Lab question on the Assignment.
Materials
- tap water
- beaker
- 25 mm × 250 mm test tubes (or larger) (one for each reaction)
- three 10-mL graduated cylinders
- test-tube clamp (or utility clamp)
- reflux apparatus (one-hole stopper with inserted glass tubing)
- alcohols and carboxylic acids made available by your teacher
- dropper bottle containing concentrated sulfuric acid (handled only by your teacher)
- weighing boat
- lab stand
- hot plate
- thermometer
- thermometer clamp
- laboratory tongs
- laboratory scoop
- electronic balance
- a vial of salicylic acid
- evaporating dish (one per reaction)
- test-tube rack
- tray or large beaker containing ice
If you have access to the materials listed, you may be able to perform this investigation.
![]() If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 4 Lab: Synthesizing Esters.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 4 Lab: Synthesizing Esters.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
Procedure
Step 1: Assemble a water bath by placing a 250-mL beaker, half-filled with tap water, onto the hot plate. Place the water bath close to the lab stand. Attach the thermometer clamp to the lab stand, and position the thermometer inside the clamp in such a way that it measures the temperature of the water inside the water bath. Attach the test-tube clamp to the lab stand.
Step 2: Transfer approximately 4 mL of the carboxylic acid you have chosen and approximately 5 mL of the alcohol you have selected into separate graduated cylinders. If you use salicylic acid, measure approximately 2 g of the salicylic acid crystals into a weighing boat.
Step 3: Transfer the contents from the graduated cylinders to the test tube. Fasten the test tube to the test-tube clamp attached to the lab stand in such a way that the reactants in the test tube are below the surface of the water bath.
Step 4: Use a clean graduated cylinder to measure 2 mL of concentrated sulphuric acid. Add this to the contents of the test tube.
Step 5: Attach the reflux apparatus to the test tube. Turn on the hot plate, and begin heating the water bath. Monitor the temperature of the water bath during the experiment to maintain the temperature of the water bath between 70 °C and 80 °C. Allow the contents of the test tube to heat for 15 minutes.
Step 6: Remove the test tube from the water bath, and allow the contents to cool for five minutes; then place the test tube into a second water bath containing cold or ice water for five minutes.
Step 7: Pour the cooled contents into an evaporating dish. Gently waft the vapour from the dish containing the synthesized ester toward you. Record the odour detected in the appropriate place in your table.
Step 8: Repeat Steps 2 to 7 for your two other esters.
Step 9: Follow your teacher’s instructions regarding the disposal of liquid waste. Be careful not to spill or splash the esters—these mixtures contain sulfuric acid.
Step 10: Disassemble, wash (if needed), and return the equipment to its appropriate place.
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
If you have not already done so, retrieve your copy of the Module 6: Lesson 4 Assignment that you saved to your computer earlier in this lesson. Complete the remainder of the Assignment and save it in your course folder. You will receive instructions later in this lesson on when to submit your completed Assignment to your teacher.
1.22. Page 4
Module 6—Petrochemicals
Atom Economy—One Way to Green Chemical Processes
atom economy: the percentage of material present in reactants that ends up in the desired product for a chemical process
A chemical process’ atom economy is important when trying to green the chemical industry. Atom economy is a percentage of the molecular mass of the product and the reactants used in creating the product. The equation to calculate atom economy is as follows:
![]()
In the calculation for atom economy, the reaction’s waste or by-products are not directly counted, but are significant. What is the waste product of an esterification reaction? How does the molecular mass of the waste product of an esterification compare to the molecular mass of other organic reactions; for example, a substitution reaction? Does the difference in the molecular mass of the by-products of these two reactions result in a significant difference in the atom economy when these two types of reactions are compared?
Consider the following worked example that calculates the atom economy for the production of methyl salicylate.
 Self-Check
Self-Check
SC 3. Calculate the atom economy for the esters made using the alcohols and carboxylic acids shown in the following table:
Reaction |
Alcohol |
Carboxylic Acid |
Ester |
|---|---|---|---|
1 |
ethanol |
ethanoic acid |
ethyl ethanoate |
2 |
propan-1-ol |
methanoic acid |
propyl methanoate |
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Reaction |
Alcohol |
Carboxylic Acid |
Ester |
|---|---|---|---|
1 |
ethanol (C2H6O) |
ethanoic acid (C2H6O2) |
ethyl ethanoate (C4H9O2) |
Molecular Mass |
46.08 g/mol |
62.08 g/mol |
89.13 g/mol |
Atom Economy |
|
||
2 |
propan-1-ol (C2H6O) |
methanoic acid (CH2O2) |
propyl methanoate (C4H8O2) |
Molecular Mass |
60.11 g/mol |
30.03 g/mol |
88.12 g/mol |
Atom Economy |
|
||
1.23. Page 5
Module 6—Petrochemicals
 Reflect on the Big Picture
Reflect on the Big Picture
Designing chemical reactions that consider atom economy can be very helpful when attempting to improve efficiency and minimize the chemical industry’s effect on the environment.
When greater proportions of a chemical reaction’s reactants end up in the product, fewer waste or by-products are made. In the calculations you just performed, the only by-product is a water molecule, which is both small and non-toxic. In general, complex syntheses involving multiple reactions have lower atom economies and lower overall yields.
 Try This
Try This
Develop an answer to one of the following questions. When possible, use examples and relevant calculations to support your answers.
TR 1. How does the molecular mass of the waste product of an esterification compare to the molecular mass of other organic reactions; for example, a substitution reaction?
TR 2. Does the difference in the molecular mass of the by-products of the two reactions in TR 1 result in a significant difference in the atom economy when these two types of reactions are compared?
TR 3. How could a chemical process be designed to maximize atom economy?
TR 4. How would the atom economy of a process that requires many reactions compare to a process that is completed in one reaction?
![]() Post your answer to the discussion area for your class. Read the responses of at least two other students. Do their responses show a correct understanding of atom economy? Share your constructive comments with your classmates. Revise your response, if necessary, and submit a copy to your teacher.
Post your answer to the discussion area for your class. Read the responses of at least two other students. Do their responses show a correct understanding of atom economy? Share your constructive comments with your classmates. Revise your response, if necessary, and submit a copy to your teacher.
 Module 6: Lesson 4 Assignment
Module 6: Lesson 4 Assignment
Submit your completed Module 6: Lesson 4 Assignment to your teacher.
1.24. Page 6
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
In this lesson you learned about carboxylic acids and esters, two classes of organic compounds.
In Lesson 4 you considered the following questions:
- What are carboxylic acids and esters?
-
How are carboxylic acids and esters involved in the petrochemical industry?
During this lesson you synthesized an ester in a laboratory investigation, and you calculated the atom economy of an esterification reaction. Many chemical industries synthesize organic compounds such as carboxylic acids and esters.
Chemical industries consider atom economy and other principles of green chemistry when developing their processes to improve their scientific and business practices and to reduce their environmental impact. In Lesson 6 you will apply your knowledge from this lesson to investigate polymers—both natural and synthetic—that are produced by esterification reactions.
Lesson Glossary
atom economy: the percentage of material present in reactants that ends up in the desired product for a chemical process
carboxylic acids: a family of organic compounds characterized by the carboxyl group (–COOH)
esterification reaction: a chemical reaction in which a carboxylic acid and an alcohol combine to produce an ester and water
esters: a family of organic compounds characterized by the ester functional group (–COO–)
1.25. Lesson 5
Module 6—Petrochemicals
Lesson 5—Addition Polymers
 Get Focused
Get Focused

© Sandra Cunningham/shutterstock
In Lesson 4 you learned that an esterification reaction involves a synthesis, or joining, of molecules. Many of the petrochemical industry’s products involve synthesis reactions—the most important example of this is plastics. In Lessons 5 and 6 you will learn how addition and condensation reactions can be used to synthesize different types of plastics.
Although many people recycle the plastics they use, estimates suggest that only five percent of the plastic created (as an off-shoot of the petrochemical industry) is recycled. This low recycling rate is due, in part, to the lack of incentive to get people to recycle plastics and the lack of a market for recycled plastics.
Not all plastics are recyclable. You can identify whether an item is recyclable by looking for the recycling code on the product. The number within the code indicates the composition of the plastic. Recyclable plastics include polyethylene, polyvinyl chloride, polypropylene, and polystyrene. You will explore these plastics in this lesson.
You may recall examining the chemical structures of these compounds in Lesson 1 of this module. What aspect of the chemistry of these substances allows them to be made into plastics?
Consider the following questions as you complete Lesson 5:
- What is the process used to make plastic?
- What properties must molecules have if they are to be used in making plastic?
 Module 6: Lesson 5 Assignment
Module 6: Lesson 5 Assignment
There is no assignment for this lesson. However, you will be asked to submit samples of your work to your teacher where instructed.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.26. Page 2
Module 6—Petrochemicals
 Explore
Explore
There is plastic in almost everything around you. Plastics are polymers—macromolecules created by linking many smaller molecules. The small molecules that make up a polymer are called monomers. The process of joining monomers together is called polymerization.
polymer: a large molecule made by linking together many smaller molecules called monomers
monomer: a small molecule that links with many other similar molecules in an addition or condensation reaction to create a polymer
polymerization: the process of forming polymers from monomers through addition or condensation reactions
 Self-Check
Self-Check
In Lesson 1 of this module you organized diagrams of the chemical structures for different petrochemical compounds based on structural similarities. In this activity you will review those diagrams to learn more about polymers. Retrieve those diagrams now.
Step 1: Sort through the diagrams and find the molecules with the term “poly” in their name.
Step 2: Sort through the remaining diagrams. For each polymer identified in Step 1, find the card that contains the corresponding monomer.
SC 1. Prepare a chart with two columns. The left-hand column should be titled “Polymer” and the right-hand column should be titled “Corresponding Monomer.” Complete the chart based on your work in Steps 1 and 2.
SC 2. Identify the similarity between all the monomers listed in your table. Suggest a mechanism by which the monomers can be joined to become a polymer.
SC 3. Sort through the remaining cards and identify any other substances that could be monomers. Justify why you chose these molecules.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Polymer |
Corresponding Monomer |
|---|---|
polyvinyl chloride |
vinyl chloride |
polystyrene |
styrene |
polypropylene |
propene |
polyvinyl acetate |
vinyl acetate |
SC 2. All monomers have a double bond between adjacent carbon atoms. The monomers would combine through an addition reaction in which the double bond is broken and each atom is saturated by bonding to another atom (in this case, the carbon atom of another monomer with a free-bonding electron).
SC 3. Other possible monomers are ethylene and butadiene. Both of these substances have double bonds and could participate in addition reactions with other monomers.
As you have seen from this Self-Check activity, there are many kinds of polymers. Polymers made from petrochemicals are often called plastics because they have the ability to be moulded. You may be familiar with other kinds of polymers, like polyester, which will be discussed in Lesson 6. As you may have guessed, polyesters have a different chemistry than addition polymers, the topic of this lesson.
 Read
Read
Read pages 445–447 in the textbook to learn more about addition reactions—one of the mechanisms by which addition polymers can be formed.
 Try This
Try This
Teflon: Healthy or Hazardous?

© Tatiana Popova/shutterstock
When Teflon was first used as a non-stick agent in cookware, it was considered revolutionary. Teflon was an exciting find for people watching their weight or cholesterol, because it allowed food to be cooked with little or no oil. Is there more to the Teflon story? Is Teflon toxic?
TR 1. Open “Web Activity: Web Quest—Teflon: Healthy or Hazardous” referenced on page 448 in the textbook. Read the instructions and discuss which parts of the project you will be responsible for with your teacher. Complete the activity. Go to the website for the textbook if you need live links to the websites and documents referenced. Information to help you find the activity on the website is available in the print version linked into this paragraph.
When you have completed the activity, contact your teacher for further instructions on submitting and/or sharing your presentation.
Save a copy of your work and your presentation in your course folder.
 Self-Check
Self-Check
SC 4. Complete “Practice” questions 1–8 on page 448 of the textbook.
 Discuss
Discuss

© Elena Schweitzer/shutterstock
Plastics form part of almost every product you use, and they have added convenience to many areas of your life. Cars, computers, cell phones, water bottles, and baby bottles all contain plastic. Even most of your food and toiletries come in plastic containers. As a result, even with recycling programs, many landfills are full of plastic.
D 1. Think about everything you have learned so far in Module 6. Do you think the way society uses plastics is a good use of the natural resource from which plastics originate? Write a few paragraphs to provide your opinion on this question.
Post your response to the discussion area for your class. Read the responses of at least two other students. Identify similarities and differences in the responses you read. Save a copy of your opinion and the similarities and differences you identified in your course folder.
1.27. Page 3
Module 6—Petrochemicals
 Reflect on the Big Picture
Reflect on the Big Picture

© anna karwowska/shutterstock
Plastics for food storage—often made of polypropylene and polystyrene—are tested for safety. Tests have shown that polypropylene can be heated to high temperatures without any leeching of monomers into the food or beverage the container holds. Heating or exposing polystyrene to alcohol or oil, however, will cause a breakdown of the polymer, which then leeches some styrene monomers into the food or beverage.
The plastics industry, a subset of the petrochemical industry, is addressing safety and environmental concerns in several ways. For example, the recycle codes on plastic products provide recycling information and tell consumers how to safely use each product. The industry is also adding a variety of molecules to increase plastic’s ability to biodegrade. In Lesson 6 you will learn about bioplastics, which can totally degrade.
 Module 6: Lesson 5 Assignment
Module 6: Lesson 5 Assignment
There is no Assignment for this lesson.
1.28. Page 4
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following questions:
- What is the process used to make plastic?
- What properties must molecules have if they are to be used in making plastic?
Polymerization is the process of creating large molecules by linking many smaller molecules together. One type of polymerization is addition polymerization. This process involves linking many alkene molecules to form polymers. Polymers are many monomers linked together. Synthetic polymers such as polyethylene, polyvinyl chloride, polypropylene, and polystyrene are used to create a variety of plastic products.
Lesson Glossary
monomer: a small molecule that links with many other similar molecules in an addition or condensation reaction to create a polymer
polymer: a large molecule made by linking together many smaller molecules called monomers
polymerization: the process of forming polymers from monomers through addition or condensation reactions
1.29. Lesson 6
Module 6—Petrochemicals
Lesson 6—Condensation Polymers

© microstocker/shutterstock
 Get Focused
Get Focused
You now know that polymers are important substances that have many uses. Addition polymers, which you studied in Lesson 5, are formed when monomers with double bonds join to make much larger molecules. Have you studied other reaction types that can also connect smaller molecules to make larger molecules?
In Lesson 4 you learned about esterification reactions, a type of condensation reaction in which a water molecule is formed when alcohol and organic acid molecules are joined together. Could this reaction type be used to make a polymer?
In this lesson you will learn how condensation polymerization reactions can be used to produce both synthetic (such as plastics) and natural polymers including proteins, lipids, and carbohydrates. You will compare many natural and synthetic polymers and their respective properties.
Consider the following questions as you complete Lesson 6:
- How do natural and synthetic polymers compare?
- What are other reaction mechanisms by which polymers can be made?
You will also take another look at how the petrochemical industry, and its offshoot industries like the plastics industry, are attempting to green their practices.
 Module 6: Lesson 6 Assignment
Module 6: Lesson 6 Assignment
Download a copy of the Module 6: Lesson 6 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in the lesson.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.30. Page 2
Module 6—Petrochemicals
 Explore
Explore
 Try This
Try This
The Structure of a Polyester

© sirano100/shutterstock
You have probably heard of polyester. Many fabrics are made from this polymer, which is formed by linking monomers with the ester functional group. In this activity you will learn more about the structure of a polyester.
Retrieve the diagrams of the chemical structures of the petrochemical compounds you started using in Lesson 1. Find the diagrams with the chemical structures for the following molecules:
- ethanol
- isopropanol
- ethylene glycol
- acetic acid
- adipic acid
TR 1. Using structural formulas, write balanced chemical equations to describe the six esters that can be formed using these components.
TR 2. Recall that a polymer is a long molecule often containing thousands of monomers. Which one of the esters you wrote in TR 1 has the potential to continue to react to form a long molecule?
TR 3. Write the balanced chemical equation for the formation of a polymer using the monomers identified in TR 2. Ensure that the polymer you show in your answer has at least two of each of the monomer molecules.
Save your work in your course folder, and submit a copy of your response to TR 3 to your teacher.
 Read
Read
condensation polymerization: a reaction in which a water molecule is produced when two monomers are chemically joined
In the Try This activity you just completed, you identified that a polymerization reaction can involve a reaction other than an addition reaction. Condensation polymers and condensation polymerization reactions are very common in industry and in biological systems. Read pages 449–452 in the textbook to learn more about condensation polymers and polymerization reactions.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 9–11 on page 451 and questions 12–14 on page 452 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 9.
The unsaturated lipids have a trigonal planar shape around the double bonds, making it more difficult for the molecules to aggregate into a solid. This results in lower melting points than those of the corresponding saturated lipids.
Practice 10.
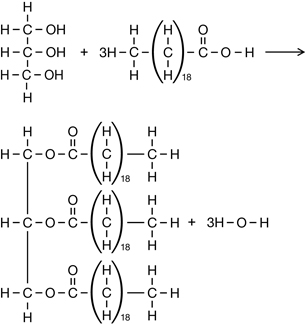
Practice 11.
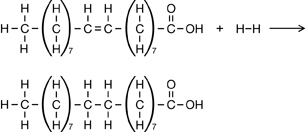
Practice 12.
Practice 13.
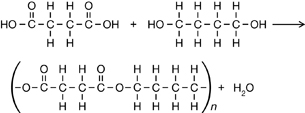
Carboxylic acid (-COOH) and alcohol (-OH) functional groups must be present to form a polyester.
Practice 14.
The reactions to form fats and to form polyesters are similar because, in both cases, linkages are formed by “condensing out” water molecules. The reactions are different in that fats are only tri-esters, whereas polyesters contain many monomer units.
 Discuss
Discuss

© 2009 Jupiterimages Corporation
Cloth or disposable diapers? This might be a decision that you have to make in the future as you become a parent. Read through “Explore an issue” on page 455 in the textbook.
D 1. In a document, indicate whether you will support cloth diapers or disposable diapers, and list supporting facts. Next, include some insight into making your product choice more green. What materials or processes could be used to make your choice of diaper more environmentally sound?
Post your response in the discussion area for your class, and respond to the opinions of at least two students who do not share your viewpoint. Save your work in your course folder.
1.31. Page 3
Module 6—Petrochemicals
 Going Beyond
Going Beyond

© Paulo Resende/shutterstock
Proteins—key components of plants and animals—are another type of natural polymer. Proteins are formed when many amino acids are joined together using an amide functional group between monomers.
proteins: natural polymers of amino acids forming the basic material of living things
amide functional group: a group of atoms composed by connecting a carboxyl group to an amine group
nylon: a synthetic condensation polymer
Nylon is a synthetic polymer that is also created using amide groups between monomers. Nylon was created as a substitute for silk and is very strong. Nylon is used to create ropes, parachutes, and as you well know, many of the clothes you wear.
 Read
Read
Read pages 452–454 in the textbook to learn more about proteins and nylon.
 Read
Read

© Smit/shutterstock
Carbohydrates are an important polymer in biological systems. Starch, cellulose, and glycogen are all polymers of sugar molecules joined by a condensation reaction.
Read pages 456–458 in the textbook to learn more about carbohydrates and the synthetic polymer cellulose acetate.
 Self-Check
Self-Check
SC 2. Complete “Practice” questions 18–23 on page 458 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Practice 18.
There are hydroxyl (-OH) functional groups present in glucose and fructose molecules.
Practice 19.
Cellulose and starch are polysaccharides. The rigid, layered structure of cellulose provides support to plants and plant cells. Because its single chains are small enough to be soluble and mobile, starch serves as a means of storing and transporting energy in plants.
Practice 20.
- Sugars are small molecules and are readily soluble because of strong intermolecular bonding with solvent water molecules. Starch is a polymer of the simple sugar glucose. While starch’s helical shape and single-strand structure allow it to be soluble, it is not as soluble as sugars.
- Cellulose has a different structure than starch. Cellulose is composed of linear polymer chains that can align side by side and favour interchain hydrogen bonding, thus creating a rigid, inflexible structure.
Practice 21.
Carbohydrate molecules can be recognized by their ring structure, which has an oxygen atom in the ring and numerous hydroxyl (-OH) groups attached to the ring.
Practice 22.
Sugar molecules form stronger intermolecular bonds than do hydrocarbons because of their polarity and ability to hydrogen bond. The shape of sugar molecules also allows them to readily fit into a crystal lattice.
Practice 23.
Glucose is highly soluble, starch is slightly soluble, and cellulose is insoluble. Glucose is highly soluble because it is a small, polar, hydrogen-bonded compound that readily forms intermolecular bonds with water molecules. Although starch is a polymer, its single chains are small enough to be water-soluble. Also a polymer, cellulose is insoluble because of its bulky, inflexible structure made of layered sheets.
 Self-Check
Self-Check
SC 3. Complete “Section 10.5” questions 1, 2, and 4–14 on page 460 in the textbook.
1.32. Page 4
Module 6—Petrochemicals
 Reflect on the Big Picture
Reflect on the Big Picture

Earlier in this module you considered concerns regarding the use of plastic shopping bags. Plastics have received a negative reputation for polluting the environment and for taking considerable time to degrade. These concerns are driving an increased interest in bioplastics. Bioplastics are a type of biopolymer—a natural polymer.
Bioplastics are made from polyalkanoates, a type of polymer containing ester bonds between monomers. Polyalkanoates are created by some plants and bacteria. Since these polyesters are naturally produced, they can also be broken down by bacteria and other organisms, making them biodegradable. Could bioplastics be the material of the future?
RBP 1. As a final reflection on what you learned in this module, retrieve the document in which you answered the “Starting Points” questions on page 410 in the textbook. Revise the answers you initially provided. Comment on how your answers changed as a result of what you learned in this module. Resave your document in your course folder.
 Module 6: Lesson 6 Assignment
Module 6: Lesson 6 Assignment
Retrieve your copy of the Module 6: Lesson 6 Assignment you saved to your computer earlier in this lesson. Complete the Assignment, and submit a copy to your teacher.
1.33. Page 5
Module 6—Petrochemicals
 Lesson Summary
Lesson Summary
In Lesson 6 you considered the following questions:
- How do natural and synthetic polymers compare?
- What are other reaction mechanisms by which polymers can be made?
There are many examples of synthetic and natural polymers that can be produced through condensation polymerization. Specifically, you considered polyester, proteins and nylon, and carbohydrates and cellulose acetate.
You also further considered the effect of the petrochemical industry and its offshoot industries on the environment, and you took a look at bioplastics.
Lesson Glossary
amide functional group: a group of atoms composed by connecting a carboxyl group to an amine group
condensation polymerization: a reaction in which a water molecule is produced when two monomers are chemically joined
nylon: a synthetic condensation polymer
proteins: natural polymers of amino acids forming the basic material of living things
1.34. Module Summary/Assessment
Module 6—Petrochemicals
 Module Summary
Module Summary
In Module 6 you considered the following module questions:
-
What kinds of chemical reactions are used to manipulate hydrocarbons in order to produce petrochemicals?
-
How has the chemical industry responded to concerns about the environment?
-
What are some of the processes that occur within Alberta’s petrochemical industry?
You explored the petrochemical industry and the processes used to convert the hydrocarbons found in petroleum into petrochemicals. Many of the products you use every day are made from petrochemicals.
The chemical structures of hydrocarbons are extremely important to the type of petrochemical that can be made from the hydrocarbons and the reaction type that will work with them. For example, you studied polymers, which can be made by either addition reactions (from unsaturated compounds) or condensation reactions (from hydrocarbon derivatives).
You also learned that it is important to consider atom economy and toxicity of reaction by-products when designing chemical syntheses—these are important considerations if the chemical industry is to demonstrate sustainability.
Concept Map or Graphic Organizer
As you worked through Module 6, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 6 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 6 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
The Module 6 Assessment is included in the Unit C Assessment. Refer to the Unit C Assessment for details.
1.35. Module Glossary
Module 6—Petrochemicals
Module Glossary
addition reaction: a type of organic reaction involving an unsaturated hydrocarbon and a small molecule, usually hydrogen or a halogen, that increases the saturation of the hydrocarbon
alcohol: an organic compound containing one or more hydroxyl groups as the functional group
amide functional group: a group of atoms composed by connecting a carboxyl group to an amine group
atom economy: the percentage of material present in reactants that ends up in the desired product for a chemical process
carboxylic acids: a family of organic compounds characterized by the carboxyl group (-COOH)
chlorofluorocarbons (CFCs): a group of organic compounds composed of carbon, hydrogen, chlorine, and fluorine
condensation polymerization: a reaction in which a water molecule is produced when two monomers are chemically joined
esterification reaction: a chemical reaction in which a carboxylic acid and an alcohol combine to produce an ester and water
esters: a family of organic compounds characterized by the ester functional group (-COO-)
functional group: a characteristic arrangement of atoms or bonds within a molecule that determines the most important chemical and physical properties of a class of compounds
hydrocarbon derivative: a molecular compound of carbon, at least one other element, and, usually, hydrogen
monomer: a small molecule that links with many other similar molecules in an addition or condensation reaction to create a polymer
nylon: a synthetic condensation polymer
organic halide: an organic compound in which one or more of the hydrogen atoms have been replaced by halogen atoms
petrochemical: a chemical made or extracted from petroleum
polymer: a large molecule made by linking together many smaller molecules called monomers
polymerization: the process of forming polymers from monomers through addition or condensation reactions
proteins: natural polymers of amino acids forming the basic material of living things
substitution reaction: a type of organic reaction involving breaking a carbon-hydrogen bond and replacing the hydrogen atom with another atom or group of atoms