Module 1
| Site: | MoodleHUB.ca 🍁 |
| Course: | Chemistry 30 SS |
| Book: | Module 1 |
| Printed by: | Guest user |
| Date: | Sunday, 14 December 2025, 10:53 AM |
Description
Created by IMSreader
Table of contents
- 1. Module 1
- 1.1. Big Picture
- 1.2. In this Module
- 1.3. Lesson 1
- 1.4. Page 2
- 1.5. Page 3
- 1.6. Page 4
- 1.7. Page 5
- 1.8. Lesson 2
- 1.9. Page 2
- 1.10. Page 3
- 1.11. Page 4
- 1.12. Page 5
- 1.13. Lesson 3
- 1.14. Page 2
- 1.15. Page 3
- 1.16. Page 4
- 1.17. Page 5
- 1.18. Page 6
- 1.19. Lesson 4
- 1.20. Page 2
- 1.21. Page 3
- 1.22. Page 4
- 1.23. Lesson 5
- 1.24. Page 2
- 1.25. Page 3
- 1.26. Page 4
- 1.27. Page 5
- 1.28. Lesson 6
- 1.29. Page 2
- 1.30. Page 3
- 1.31. Page 4
- 1.32. Lesson 7
- 1.33. Page 2
- 1.34. Page 3
- 1.35. Page 4
- 1.36. Lesson 8
- 1.37. Page 2
- 1.38. Page 3
- 1.39. Module Summary/Assessment
- 1.40. Module Glossary
1. Module 1
Module 1—Thinking Energy
Module Introduction

© 2008 Jupiterimages Corporation
Energy is defined as the capacity of a substance or system to do work. Energy—including the development and export of natural resources such as coal, natural gas, oil, and bitumen from oil sand—is an important part of Alberta’s economy. Energy is also an important part of your daily life. You rely heavily on fossil fuels to provide energy to heat your home and to power the machinery, equipment, electronics, and more that are part of almost every part of your life.
Energy can come in a variety of forms. Almost all of the events that occurred earlier in your day today involved energy in some form. Your alarm clock’s conversion of electrical energy into sound energy may have woken you up. Your water heater’s transformation of chemical potential energy from natural gas or another fuel into thermal energy probably provided the warm water for your shower. In this module you will investigate the quantity of energy involved in your daily activities.
Module 1 will focus on various energy sources and will consider those sources within the context of providing energy for the activities you enjoy. You will learn ways to measure and calculate energy change during chemical reactions.
In Module 1 you will investigate the following questions:
- Should energy be given a higher priority when making decisions about society’s future?
- How does society use the energy of chemical changes?
- What are the impacts of energy use on the environment?
- How does society use knowledge of the energy associated with chemical processes to promote sustainability?
- In what ways have issues of energy use affected the development of past and present societies?
Remember that each lesson will also be organized around questions intended to guide your study. As you proceed through Module 1, you may record answers to these questions and any interrelationships that exist between them in a concept map or graphic organizer. More information is available in the Unit A Concept Organizer. In the Module 1 Summary you will receive further information on how you can use your concept map or graphic organizer to review the concepts you studied in this module.
1.1. Big Picture
Module 1—Thinking Energy
 Big Picture
Big Picture

© Jan Martin Will /shutterstock
Want to see the North? Canada's northern-most regions are becoming tourism hotspots. Before visiting the Arctic you will want to make sure your visit will not harm the area's delicate ecology. You might consider “going eco” on your next holiday!
Ecotourism—the opportunity to visit and observe natural habitat and wildlife without leaving a negative ecological footprint—is the new rage. Ecotourism encourages the use of sustainable resources and the promotion of sustainable development within the area visited. It also focuses on minimizing the impact of visitors on the wilderness showcased.
Ecotourism can provide for many unique holidays ranging from observing wildlife such as wolves, beluga whales, muskoxen, and caribou; to more energetic pursuits like canoe trips, whale watching in kayaks, travel by komatik, cross-country skiing, snow shoeing, and hiking. Ecotour accommodations vary from tents on the tundra to cozy lodges with gourmet meals.
When planning and delivering ecotours, ecoguides and tour operators must make many logistical decisions including how their tour participants will move around and what fuel will power any vehicles participants use.
In Module 1 you will plan an ecotour of the area in which you live. You will consider how thermodynamics can be applied to the type of equipment you will use and the practices you will follow during your ecotour. In your role as an ecotour operator you will evaluate what priority should be given to energy consumption when making decisions about your ecotour company and society's future.
As you develop a plan for your ecotour, you will consider the unique ecological, geological, or geographic aspects of your part of Alberta. Depending on where you live, you may develop a tour that features the stunning Cypress Hills or Rocky Mountains, the many lakes in the Lac La Biche area, or the many ways to navigate Edmonton's river valley.
Your ecotour planning will tie into the Module 1 Assessment. Read on to learn more about the lessons, activities, and assessments you will complete as you progress through Module 1.
 Assessment in This Module
Assessment in This Module
Each lesson contains a range of activities and assessment options. These include assignments, labs, and Self-Check, Try This, Discuss, Reflect and Connect, and Reflect on the Big Picture activities. Instructions will be provided for each of these activities so that you can appropriately focus your time and effort. Your teacher will tell you which assessment options to complete and which responses to submit for marks or feedback. Remember to save all of your work in your Chemistry 30 folder.
The following are examples of the activities you will complete:
- Choice of Snack Foods (Lesson 2)
- Choice of Sleeping Bag (Lesson 3)
- Recharging Hot Packs (Lesson 4)
- Evaluating Different Fuels (Lesson 5)
- Evaluating Two Liquid Fuels (Lesson 6)
- Reflect on the Big Picture (Lesson 8)
For your Module 1 Assessment, you will submit your responses to your choice of four of the above activities as part of the planning component of your ecotour. You will also develop an advertising brochure to describe and attract tourists to the tour you are providing.
More information about the components of your ecotour plan is provided in the lessons and in the Module Assessment. You may wish to look at the Module Assessment and the Unit Assessment before starting Lesson 1.
1.2. In this Module
Module 1—Thinking Energy
In This Module
Lesson 1—Personal Energy Use
In Lesson 1 you will consider how your body and other biological systems use energy. You will learn about the origin of the energy in the food you eat. You will review the processes of photosynthesis, cellular respiration, and the carbon cycle; and you will consider their importance to the energy sources used by society.
You will investigate the following lesson questions:
- How does society use the energy of chemical changes?
- How do you use the energy of chemical changes in your life?
Lesson 2—How Much Energy?
How is the energy content of a food determined? In Lesson 2 you will use calorimetry to quantify energy associated with a chemical process or a food. You will use knowledge gained in this lesson to select foods to meet the energy requirements of clients in your ecotourism company.
You will investigate the following lesson question:
- How is energy quantified?
Lesson 3—Specific Heat Capacity
In Lesson 3 you will look at an important thermodynamic property unique to each substance-specific heat capacity. You will use your knowledge of specific heat capacity to evaluate materials with respect to their insulating properties.
You will investigate the following lesson question:
- How can knowledge of specific heat capacity be used in the design of a sleeping bag?
Lesson 4—Enthalpy and the Kinetic Molecular Theory
In Lesson 4 you will consider the changes in energy that are associated with chemical change.
You will investigate the following lesson questions:
- What changes occur during a chemical reaction at the particle level?
- Why do chemical changes and energy changes occur at the same time?
- What is enthalpy?
Lesson 5—Molar Enthalpy
In Lesson 5 you will learn more about enthalpy. You will understand the importance of knowing the enthalpy change for a chemical reaction per mole of reactants involved and how this may be used to evaluate different fuels used by your ecotourism company.
You will investigate the following lesson question:
- How can the energy richness of different fuels be compared?
Lesson 6—Communicating Enthalpy Change
In Lesson 6 you will learn different ways to communicate enthalpy changes for a chemical reaction.
You will investigate the following lesson question:
- How are enthalpy changes communicated?
Lesson 7—Hess’ Law
In Lesson 7 you will learn to use Hess’ law, an alternative method to calculate enthalpy change for a system without using a calorimeter. You will consider why this method might be used instead of obtaining a value through experimentation.
You will investigate the following lesson questions:
- What is Hess’ law?
- How does Hess’ law relate to enthalpies of reaction?
Lesson 8—Molar Enthalpy of Formation
In Lesson 8 you will use standard molar enthalpies of formation to calculate the enthalpy change for a chemical reaction.
You will investigate the following lesson question:
- How are standard molar enthalpies of formation used to determine the enthalpy change associated with chemical reactions?
1.3. Lesson 1
Module 1—Thinking Energy
Lesson 1—Personal Energy Use
 Get Focused
Get Focused

© RJR/shutterstock
In this lesson you will investigate the energy changes that occur during the chemical reactions involved in photosynthesis, cellular respiration, and combustion. You will learn how these reactions are part of the carbon cycle.
As you work through the lesson, you will be planning an ecotour. As an ecotour operator, you are responsible for your clients and for their impact on the environment in which your tour operates. Planning your ecotour will require you to consider how energy changes relate to situations outside of a chemistry laboratory.
You will need to keep your clients well-fed by offering them appropriate foods. While there are many qualities to consider when selecting food, in this case you will focus on the energy content of food. To get an idea of how much food your clients will need, you will do an inventory of and evaluate your own personal energy intake and needs.
Energy is involved in every activity. In this lesson you will investigate the quantity of energy you intake to support your daily activities.
Consider the following questions as you complete Lesson 1:
- How does society use the energy of chemical changes?
- How do you use the energy of chemical changes in your life?
 Module 1: Lesson 1 Assignment
Module 1: Lesson 1 Assignment
In the Lesson 1 Assignment you will prepare a spreadsheet to calculate your personal energy intake. Download a copy of the teacher-marked Module 1: Lesson 1 Assignment to your computer at this time. You will receive further instructions on how to complete this assignment later in the lesson.
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check, Try This, and other types of questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons. You should record the answers to all the questions in the lesson and place those answers in your course folder.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit the responses to Try This and other questions that are not marked to your teacher for informal assessment and feedback. Your answers are very important to your teacher. They provide your teacher with information about your learning, and they help your teacher identify where adjustments to your instruction may be necessary.
1.4. Page 2
Module 1—Thinking Energy
 Explore
Explore
Ultimate Energy Sources
 Self-Check
Self-Check
Combustion processes are used by many cultures to obtain energy. Refer to the following pictures to answer the Self-Check questions below.

left: © Péter Gudella /shutterstock right: R. Knights/NWT Archives
SC 1. The photos above illustrate three combustion processes. Where does the energy within each fuel come from?
SC 2. Draw a diagram that traces the energy source in each of the fuels back to its origin. Save a copy of the diagrams in your course folder. You may wish to refer to these later.
SC 3. State two additional ultimate energy sources?
 Self-Check Answer
Self-Check Answer
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. All energy sources originated from biological sources (wood, coal, and animal fat), meaning that the ultimate source of energy was the sun.
SC 2. Your diagrams should look like the following (arrows indicate the direction of the flow of energy):
sun → tree → firewood
sun → plant/animal tissue → coal
sun → plant life → fish → seal oil
Remember to place a copy of these diagrams in your course folder for a later look.
SC 3. The other ultimate energy sources include geothermal energy and gravitational energy.
 Read
Read
Read pages 480 and 481 in the textbook, Nelson Chemistry Alberta 20–30.
Compare your answers to SC 1 and SC 2 with what you read in the textbook. Did you correctly identify the original source of energy for the three fuels? Modify your answer if you need to, and then place an updated copy in your course folder.
 Read
Read
chemical potential energy: the form of energy present within the chemical bonds of a substance
What purpose does food have? Although taste is important, food is all about energy. How much energy do you require to perform your daily activities? Why are you hungrier after a hike in the mountains than when you are sitting and watching television?
To perform your daily activities, your body uses chemical potential energy from the food you consume. In previous science courses you learned that energy is measured in joules. The energy in food can also be measured in another unit, calories.
Read the “Personal Use of Chemical Energy” case study on page 482 of the textbook. In the case study, you will investigate how you obtain energy from your diet and how energy intake should be balanced with activity level and other energy requirements.
 Self-Check
Self-Check
SC 4. Complete question 1 of the case study on page 482 in the textbook.
SC 5. Complete question 2 of the case study on page 482 in the textbook.
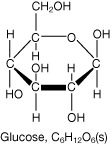
SC 6. Calculate the mass of glucose that would need to be consumed to meet the daily energy requirements of a normally active young adult. Use the values shown in the case study and the diagram of the glucose molecule.
SC 7. Repeat the calculations for a high-performance athlete.
 Self-Check Answer
Self-Check Answer
Contact your teacher if your answers vary significantly from the answers provided here.
SC 4.
Food and fossil fuels are the two main sources of chemical energy for personal use.
SC 5.
C6H12O6(s)
![]()
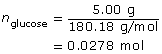
![]()
SC 6.
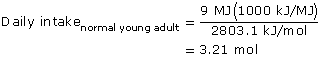
![]()
SC 7.
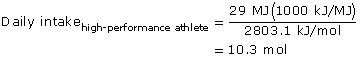
![]()
 Read
Read
Can you imagine having a diet in which you ate only glucose? A healthy diet contains other foods and energy sources.
Certain foods have more chemical potential energy than other foods. If you examine the diagram of the glucose molecule, you will identify a number of chemical bonds. The chemical potential energy you obtain from food is stored in the chemical bonds that hold molecules together. Your digestive system is designed to break these chemical bonds and to absorb or convert what you eat into carbohydrates, proteins, and fats that your body can access when it needs energy.
(e.g., glucose, C6H12O6)
Some types of chemical potential energy are easier for your body to use than others. Simple carbohydrate molecules, such as sugars like glucose, are a quick source of energy for the body because the molecule's bonds are easy to break. It takes the body longer to break the bonds in complex carbohydrates, like the starch found in pasta and whole-grain flour. Because they can provide a source of energy for a long period of time, complex carbohydrates are better for high-energy sports with long durations—hiking and cross-country skiing, for example.

© 2008 Jupiterimages Corporation
Hikers and campers use information about different types of food to make informed choices when they pack for trips. They choose foods that pack a high-energy bang for the buck. Energy bars, which are a popular choice for many hikers, often include a mixture of simple and complex carbohydrate sources. These sources provide the immediate and long-term energy to support an activity.
1.5. Page 3
Module 1—Thinking Energy
 Going Beyond
Going Beyond
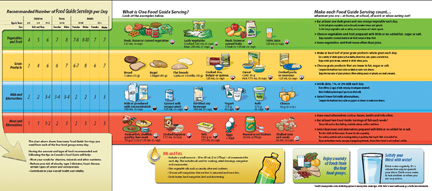
The Canada Food Guide identifies types of foods and suggested quantities for consumption.
Food is more than just calories and kilojoules. The updated Canada Food Guide focuses on serving size and the number of servings eaten per day. In Modules 5 and 6 you will learn more about the carbohydrates, proteins, and fats contained in foods. You will also discover how these dietary components perform vital functions for your body.
You may wish to use the Internet to obtain a copy of the Canada Food Guide or to find dietary information for athletes. This information will help you learn more about how appropriate nutritional and dietary choices can affect the health of your ecotour participants.
 Module 1: Lesson 1 Assignment
Module 1: Lesson 1 Assignment
How much energy do you consume each day by eating?
Retrieve the Module 1: Lesson 1 Assignment that you saved to your computer earlier in this lesson. You will record your personal energy intake in a spreadsheet. Complete the Assignment and save it in your course folder. You will receive information later in this lesson on when to submit your Assignment to your teacher.
The Sun—The Ultimate Energy Source

© Jan Brons/shutterstock
Regardless of what food you eat, the energy you consume originally came from the sun. Solar energy is converted into chemical potential energy by plants as they photosynthesize. The energy contained within plants and/or animals is captured by living organisms when the plants and/or animals (the food) undergo a chemical reaction like digestion or combustion.
hydrocarbon molecule: a chemical compound containing only hydrogen and carbon atoms

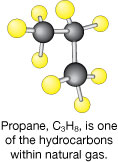
© pixelman/shutterstock
Coal is one type of fossil fuel composed of hydrocarbon molecules.
Fossil fuels, such as coal, petroleum, natural gas, and oil sand, are an important energy source. Fossil fuels contain hydrocarbon molecules. Hydrocarbons typically tend to be high-energy molecules, since the chemical bonds between carbon atoms and between hydrogen and carbon atoms store a relatively large quantity of potential energy. Burning a fossil fuel releases the energy stored in these high-energy bonds. In your home, the combustion of fossil fuels can be used for many purposes, including heating your home.
 Try This
Try This
An Analysis of the Carbon Cycle
The carbon cycle is a summary of the major reactions involving carbon compounds in the biosphere. You may have seen a diagram of the carbon cycle in previous science courses. In this activity you will complete an analysis of a diagram of the carbon cycle.
Procedure
Step 1: Print a copy of the carbon cycle diagram. Examine the diagram to refresh your memory of the carbon cycle and its processes.
Step 2: Prepare a chart listing similarities and differences between the following chemical processes, which are part of the carbon cycle: photosynthesis, cellular respiration, and combustion.
Include an example of a chemical reaction for each process in your chart. You may wish to use the Internet or other resources to find information.
Step 3: Examine the diagram of the carbon cycle. Determine the correct position on the diagram for each term you researched in Step 2.
TR 1. Write a brief paragraph to identify the important aspects of the carbon cycle.
TR 2. How is a knowledge of the carbon cycle important to your efforts to design an ecotour that will minimize impacts on the environment?
![]() Post your answers to questions TR 1 and TR 2 in the discussion area for your class. Read the answers from at least two other students. If you wish, revise your answer. Place a copy of your revised answers in your course folder.
Post your answers to questions TR 1 and TR 2 in the discussion area for your class. Read the answers from at least two other students. If you wish, revise your answer. Place a copy of your revised answers in your course folder.
1.6. Page 4
Module 1—Thinking Energy
 Reflect and Connect
Reflect and Connect

top left: © cappi thompson /shutterstock top right: © Philip Lange /shutterstock
bottom: left: © Scott T. O'Donnell/shutterstock bottom right: © Alan Freed /shutterstock
All forms of transportation require energy. What forms of energy and energy sources are necessary in the forms of transportation shown in the graphic? What relationship do these forms of energy have to the sun? If you were planning an ecotour of your local area, which forms of transportation would best fit your tour's needs? Why would you have to rely on those forms of energy? Is there a way to make transportation in your area less harmful to the environment?
RC 1. Consider these questions and others you may have thought of as you worked through this lesson. Write answers or sketch notes to yourself. Create a sub-folder titled "ecotour planning" in your course folder. Save your answers and notes in this new sub-folder. You will refer back to this sub-folder throughout Module 1.
 Reflect on the Big Picture
Reflect on the Big Picture
Ecotourism provides an opportunity to preserve natural areas by promoting sustainable travel. Ecotourists want to learn and experience the resources of an area but do not want to adversely affect the area's environment. In this lesson you began to consider some of the aspects of an ecotour. You will continue to consider other aspects of ecotourism in the coming lessons.
Use this opportunity to make some initial plans for your ecotour.
- What is unique about the area in which you live? How could this be the foundation for an ecotour?
- What locations will you visit in your ecotour?
- What activities will you do while you are at these locations?
- How will the participants in your ecotour get to and from these locations?
- How will your participants explore the different regions you take them to?
- Can you make the tour accessible to people with disabilities and, if so, what changes might you have to make to your plans?
Estimate the activity level of the participants in your ecotour. Use that information and the information you gathered about your own personal energy intake and requirements in your Lesson 1 Assignment to identify the foods you will need to meet your participants' energy needs.
Place your brief answers to these questions and any other ideas you have in your ecotour planning sub-folder.
 Try This
Try This
Buying carbon offsets has become an option for many travellers.
© Air Canada 2007
TR 3. What is a carbon offset?
TR 4. Is offering a traveller the opportunity to purchase a carbon offset consistent with the principles of ecotourism?
TR 5. Does allowing a traveller the opportunity to buy a carbon offset make this person more conscious of society's choices regarding energy use?
Place your answers to these questions in your ecotour planning sub-folder. You will be asked to review the information in this sub-folder as you finalize your ecotour plans later in Module 1. For example, you may use the information you gather on carbon offsets to determine whether your tour will include the option to purchase a carbon offset.
 Module 1: Lesson 1 Assignment
Module 1: Lesson 1 Assignment
Submit your completed Module 1: Lesson 1 Assignment to your teacher.
1.7. Page 5
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 1 you considered the following questions:
- How does society use the energy of chemical changes?
- How do you use the energy of chemical changes in your life?
Society uses a variety of energy sources. Greater consideration has recently been given to the environmental impact of energy use. In this lesson you learned about a variety of energy sources. These included chemical potential energy in the food you eat, the fossil fuels used for many modes of transportation, and solar energy, which is the ultimate energy source for the biosphere. You learned about the chemical reactions involved in photosynthesis, cellular respiration, and combustion; and you learned how these reactions are part of the carbon cycle.
You also assessed your personal energy intake to get an idea of how much chemical potential energy your body needs to sustain itself. You used this information to start planning your ecotour.
Lesson Glossary
carbohydrate: a carbon compound that contains many hydroxyl groups and has the general chemical formula CnH2nOn (e.g., glucose, C6H12O6)
chemical potential energy: the form of energy present within the chemical bonds of a substance
hydrocarbon molecule: a chemical compound containing only hydrogen and carbon atoms
1.8. Lesson 2
Module 1—Thinking Energy
Lesson 2—How Much Energy?
 Get Focused
Get Focused

In Lesson 1 you identified your daily energy intake from food. The food you eat provides you with chemical potential energy to maintain your body functions and your activity level. Although foods can vary greatly in the quantity of energy they provide, the energy contained within all food can be traced back to the sun—the biosphere’s ultimate energy source.
The initial planning you completed for your ecotour in Lesson 1 probably indicates that your tour participants will be active. They will need to replace the energy they burn completing the many activities you have planned for them. You may recall calculating the energy required to complete some work in a previous science course, but how is the energy in food determined? How can chemical potential energy in food be measured?
In this lesson you will investigate how the energy content of food is determined and how the principles used to collect energy are applied to other technologies. You will use what you learn to create a list of foods to offer the participants in your ecotour.
Consider the following question as you complete Lesson 2:
- How is energy quantified?
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Download a copy of the teacher-marked Module 1: Lesson 2 Assignment to your computer at this time. You will receive further instructions on how to complete this assignment later in this lesson.
In this lesson, you will also receive further instructions on, and you will complete, part of the Module 1 Assessment: Choice of Snack Foods.
You must decide what to do with the questions that are not marked by your teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You may decide to respond to all the questions and place those answers in your course folder.
1.9. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Try This
Try This
Ranking Foods by Their Energy Content

Step 1: Find at least ten foods in your home.
Step 2: Predict the amount of energy contained in one serving of each food by ranking the foods from highest to lowest energy content.
Step 3: Use the nutrition label or the Internet to determine the energy content of each food. If the energy content is given in calories, multiply the number of calories by 4.19 and record the value as kilojoules (kJ) in your table.
Analysis
TR 1. Calculate the energy content per gram for each food. Rank the foods from highest to lowest energy content per gram.
TR 2. Compare your ranking of predicted energy content with your ranking based on calculated values. Explain any differences.
 Read
Read
calorimeter: an isolated system in which the chemical system being studied is surrounded by a known quantity of liquid
calorimetry: the technological process of measuring the energy changes of an isolated system
How is the energy content of food determined? Read pages 485–489 in the textbook to learn more about a calorimeter and calorimetry.
 Watch and Listen
Watch and Listen
To understand how thermochemistry is used to determine the quantity of energy available in food, read “Food Energy.” This is a transcript of an audio clip available on the website for the textbook. To listen to the audio clip, go to www.science.nelson.com and follow the links to Unit 6, Chapter 11, Section 11.2, Extension. You may need to ask your teacher for a username and password to access the audio clip.
In the audio clip and transcript, reference is made to a more sophisticated type of calorimeter, a bomb calorimeter. View the animation “The Oxygen Bomb Calorimeter” for more information about a bomb calorimeter.
1.10. Page 3
Module 1—Thinking Energy
 Lesson 2 Lab: Constructing and Testing a Calorimeter
Lesson 2 Lab: Constructing and Testing a Calorimeter
A simple calorimeter can be used to determine the energy content of a nut, a type of food commonly found in trail mix.
![]() Read the exploration “Burning Oil” on page 479 in the textbook.
Read the exploration “Burning Oil” on page 479 in the textbook.
![]() Then, view the
virtual investigation “Lesson 2 Lab: Calorimetry.”
Then, view the
virtual investigation “Lesson 2 Lab: Calorimetry.”
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Retrieve your copy of the Module 1: Lesson 2 Assignment that you saved to your computer earlier in this lesson. Use the information from the multimedia presentation to complete Part 1: Calorimetry Lab. You will receive information later in the lesson on when to complete the rest of the Lesson 2 Assignment and on when to submit your work to your teacher.
1.11. Page 4
Module 1—Thinking Energy
 Going Beyond
Going Beyond
Calorimetric data about the energy content in foods is not always reliable. Use the Internet to research other methods used to determine the energy content in foods. Why are other methods used? Do the other methods have limitations or do they provide greater accuracy than calorimetric data?
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1 to 8 on page 487 in the textbook.
 Self-Check Answer
Self-Check Answer
SC 1.
- Energy cannot be created nor destroyed.
- All energy comes from some other form of energy.
Practice 3.
Practice 4.
- If the mass of material undergoing temperature change is doubled and the other variables remain the same, then the thermal energy is doubled.
- If the specific heat capacity of material undergoing temperature change is divided by two (is halved) and the other variables remain the same, then the thermal energy is halved.
- If the temperature change of the material is tripled and the other variables remain the same, then the thermal energy is tripled.
- If all three variables (m, c, and Δt) are doubled, then the thermal energy is increased by a factor of 8 (2 × 2 × 2 = 8).
Practice 6.
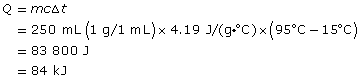
The gain in the water’s thermal energy is 84 kJ.
Practice 7.
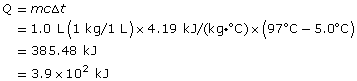
The gain in the water’s thermal energy is 3.9 x 102 kJ.
Practice 8.
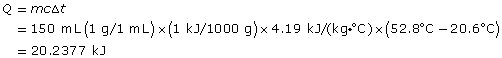
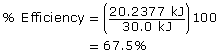
The calorimeter is 67.5% efficient.
Evaluate the Design of a Solar Panel
 Watch and Listen
Watch and Listen

Natural Resources Canada
Drake Landing is an innovative neighborhood located in Okotoks, Alberta. Part of Drake Landing’s innovation involves the way the development uses solar energy to greatly reduce the neighbourhood’s dependence on fossil fuels. As shown in the picture, energy from the sun is used to heat homes. This greatly reduces the need for fossil fuels.
View the video “Drake Landing Solar Community” for more information on how a community solar heating system works.
The solar panels found on the roofs of buildings within the Drake Landing Solar Community are part of an active solar heating system. The circulation of antifreeze solution through the system allows for the transfer of energy from the collector to the house or to the borehole energy storage system.
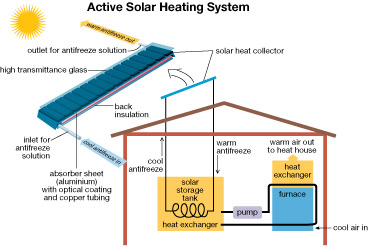
This is a schematic representation of a solar heating system and its components.
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Retrieve your copy of the Module 1: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will use your knowledge of energy transfers and of how an energy-measuring device such as a calorimeter works. Complete Part 2—Evaluate the Design of a Solar Collector, and save your work in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture

© 2000-2007, It’s Our World Recycling. Adapted with permission. <www.iowr.ca>
As you work through Module 1, you are developing a plan for an ecotour. You have been saving related work to your ecotour planning sub-folder. Ecotourism is defined as “the responsible travel to natural areas that conserves the environment and improves the well-being of local people” (The International Ecotourism Society). At the start of Lesson 2 you investigated the energy content of different foods. What foods should the participants in your ecotour eat to maintain their energy and nutrition levels?
Choice of Snack Foods
RBP 1. Prepare a list of snack foods that you intend to provide during the ecotour you are planning. Include the energy content of each food, and justify the food's use. Identify what you need to consider when selecting and using these products, and show how your use of these foods supports the principles of ecotourism. Place a copy of your list and supporting information in your ecotour planning sub-folder.
This is the first of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Submit your completed Module 1: Lesson 2 Assignment to your teacher.
1.12. Page 5
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 2 you considered the following question:
- How is energy quantified?
You learned about calorimetry, which is an experimental technique used to measure energy transfers. You used the formula Q = mcΔt to calculate the energy gain or loss by a system in order to determine the energy content of foods. You also investigated the design of calorimeters. And you applied your understanding of the principles of energy transfer to the operation of a solar collector.
Lesson Glossary
calorimeter: an isolated system in which the chemical system being studied is surrounded by a known quantity of liquid
calorimetry: the technological process of measuring the energy changes of an isolated system
1.13. Lesson 3
Module 1—Thinking Energy
Lesson 3—Specific Heat Capacity
 Get Focused
Get Focused

© Jakub Cejpek/shutterstock
One of the essential pieces of gear for an ecotourist is a sleeping bag suited to the climate. A sleeping bag that is too warm will cause you to sweat, and one that makes you feel too cool will result in you shivering through the night. Sleeping bags are rated according to the materials used in their construction and can be suitable for temperatures ranging from −40°C to +15°C.
A sleeping bag is designed to insulate a person by attempting to prevent an energy exchange between the person and the surroundings. In Lesson 2 you learned about the design of a calorimeter. It might sound like the insulating characteristics of a sleeping bag are similar to those of a calorimeter—both are designed to prevent an exchange of thermal energy between the "contents" and its surroundings.
In this lesson you will test a calorimeter that you construct using data collected while performing an experiment. You will also investigate the specific heat capacity of substances and relate this property to the selection of materials that may be used to design a sleeping bag.
Consider the following question as you complete Lesson 3:
- How can knowledge of specific heat capacity be used in the design of a sleeping bag?
 Module 1: Lesson 3 Assignments
Module 1: Lesson 3 Assignments
Download a copy of the Module 1: Lesson 3 Assignment to your computer at this time. You will receive further instructions on how to complete this assignment later in the lesson. In this lesson, you will also receive further instructions on and you will complete part of the Module 1 Assessment: Choice of Sleeping Bag.
You must decide what to do with the questions that are not marked by your teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.14. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
Calorimetry is based on two scientific principles, the first and second laws of thermodynamics:
- Energy can be neither created nor destroyed.
- Heat naturally transfers from warmer to cooler objects.
 Try This
Try This
TR 1. Look at “Figure 3” on page 486 in the textbook. Write an explanation of how the two laws of thermodynamics are shown in this diagram.
![]() Post your explanation to the discussion area for your class. Read and post comments on the explanations made by two of your classmates. Place a copy of your original explanation and any comments you receive from your classmates in your course folder.
Post your explanation to the discussion area for your class. Read and post comments on the explanations made by two of your classmates. Place a copy of your original explanation and any comments you receive from your classmates in your course folder.
TR 2. Prepare a sketch of “Figure 3.” Add labels and captions to your sketch that explain how the design of a sleeping bag employs thermodynamic principles to ensure that the user is comfortable while sleeping outdoors. Submit a copy of your diagram to your teacher for feedback.
 Watch and Listen
Watch and Listen
Energy transfers are essential to the operation of a calorimeter. Watch the video “Energy Transfers” to view an experiment measuring energy transfers between a cold metal object and the water in a calorimeter. You will use the information from this video to answer Self-Check questions 1 and 2. You may wish to read the Self-Check questions before viewing the video.
 Self-Check
Self-Check
Answer these Self-Check questions after viewing the video “Energy Transfers.”
quantitative: an observation that uses a numeric value
magnitude: a value that indicates size or quantity
SC 1. Record observations that could be used to quantitatively demonstrate that an energy transfer has occurred.
SC 2. What is the magnitude of this energy change?
Check your work.
 Self-Check Answer
Self-Check Answer
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Data Table
| mass of empty cups | 4.39 g |
| mass of cups and water | 103.39 g |
| mass of water | 99.0 g |
| final temperature of water | 19.0°C |
| initial temperature of water | 23.9°C |
The decrease in the temperature of the water in the cup indicates that energy has been transferred from the water to the metal object placed in the water.
The magnitude of the energy change can be calculated using the formula Q = mcΔt, where
Q is the quantity of energy transferred by the water
m is the mass of the water in the system involved in the energy transfer
c is the specific heat capacity of water
Δt is the temperature change for the water in the system
SC 2.
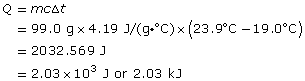
The magnitude of the energy change for the water is 2.03 kJ.
1.15. Page 3
Module 1—Thinking Energy
 Read
Read
specific heat capacity: a physical property of matter
It is the energy required to raise the temperature of one gram of a substance one degree Celsius.
Specific heat capacity is the energy required to change the temperature of one gram of a substance one degree Celsius. As you watched the video “Energy Transfers” you observed that the water in the calorimeter decreased in temperature. Assume that the piece of metal placed into the calorimeter in the video was allowed to cool to –20.0°C in a freezer. Given this information and the data you collected from the video, could you determine the specific heat capacity of the metal object?
 Self-Check
Self-Check
SC 3. Describe how you would use the data to determine the specific heat capacity of the metal object.
 Self-Check Answer
Self-Check Answer
SC 3.
Go to the “Specific Heat Capacity Worked Example—Part 1” to see the answer for this question.
SC 4. Use the data from the experiment to calculate the value for the metal’s specific heat capacity.
 Self-Check Answer
Self-Check Answer
SC 4.
Go to the “Specific Heat Capacity Worked Example—Part 2” to see the answer for this question.

© 2008 Jupiterimages Corporation
A sleeping bag is given a comfort rating, which is the lowest temperature limit at which the bag provides adequate insulation. Data from two sleeping bags are shown.
Insulating Material |
down | polyester |
|---|---|---|
| Comfort Rating | −7°C | −7°C |
| Weight | 1.35 kg | 1.37 kg |
How do you think the two bags compare in terms of their specific heat capacities? Can you identify any assumptions involved in your prediction?
Although sleeping bags can appear to have similar heat capacities and insulating properties, their compositions can be very different. The style, the materials used in construction, and the method of assembly can influence the insulating properties and the comfort rating of sleeping bags.
 Reflect and Connect
Reflect and Connect
Thermal energy transfers between warmer and cooler objects until both objects reach the same temperature. The function of a sleeping bag, or the clothes you wear, is to contain the air around your body that has been warmed from heat your body has emitted. Materials that provide better insulation from the cold tend to prevent trapped air that has been heated by the body from escaping. It is common for winter campers to place a vapour bag within a sleeping bag. The vapour bag fits inside the sleeping bag like a sleeve and is designed to transfer perspiration (water) away from the air surrounding the body.
RC 1. The specific heat capacity of water is 4.19 kJ/(kg•°C), while the specific heat capacity of air is 1.01 kJ/(kg•°C). Use this data to explain how using a vapour bag can increase a person's comfort in a sleeping bag.
![]() Post your answer to the discussion area for your class. Review the explanations provided by other classmates. Did all students have a similar understanding of how the difference in the value for specific heat capacity between water and air would affect the sleeping bag’s insulating ability? Place a copy of your posting and your comments in your course folder.
Post your answer to the discussion area for your class. Review the explanations provided by other classmates. Did all students have a similar understanding of how the difference in the value for specific heat capacity between water and air would affect the sleeping bag’s insulating ability? Place a copy of your posting and your comments in your course folder.
1.16. Page 4
Module 1—Thinking Energy
 Lesson 3 Lab: Constructing and Testing a Calorimeter
Lesson 3 Lab: Constructing and Testing a Calorimeter
Background Information
In Lesson 2 you learned about bomb calorimeters and how they are used to measure the energy content of food. Bomb calorimeters are complicated technical devices and are not available in most high school science laboratories. In this investigation you will construct and determine the precision of a simpler version of a calorimeter.
Purpose
In this investigation you will construct and determine the precision of a calorimeter.
Problem
What is the precision of the calorimeter tested as determined by percent experimental error?
![]() Retrieve your copy of the Module 1: Lesson 3 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 3 Assignment.
Retrieve your copy of the Module 1: Lesson 3 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 3 Assignment.
Materials
- four polystyrene cups
- thermometer
- electronic balance
- water
- kettle
If you have access to the materials listed, you may be able to perform this investigation.
![]() If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 3 Lab: Constructing and Testing a Calorimeter.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 3 Lab: Constructing and Testing a Calorimeter.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
Procedure
Step 1: Construct a calorimeter by nesting two polystyrene cups.
Step 2: Make a hole in the bottom of the third polystyrene cup. A thermometer will be inserted into this hole later.
Step 3: Use the electronic balance to measure and record the mass of the calorimeter, including the lid.
Step 4: Remove the calorimeter from the balance. Add room temperature water to the calorimeter until it is just less than half full.
Step 5: Use the electronic balance to measure and record the mass of the half-full calorimeter, including the lid.
Step 6: Place the lid on the calorimeter and use the thermometer to measure the water temperature in the calorimeter.
Step 7: Use the kettle to prepare warm water with a temperature of approximately 60 °C.
Step 8: Add warm water from the kettle to the fourth cup until it is just less than one-third full.
Step 9: Use the electronic balance to measure and record the mass of the fourth cup containing the warm water.
Step 10: Place the lid on the calorimeter and use the thermometer to measure the temperature of the warm water.
Step 11: Transfer the contents of the fourth cup to the calorimeter. Attach the lid to the calorimeter. Insert the thermometer through the hole made in Step 2, and use the thermometer to gently mix the contents of the calorimeter. While mixing the contents, observe the temperature change and record the highest temperature for the calorimeter contents.
Step 12: Use the electronic balance to measure and record the mass of the fourth cup.
Step 13: Empty the contents of the calorimeter, dry the inside of the calorimeter, and repeat Steps 4–12 to obtain data for nine more trials.
Step 14: Clean each apparatus used, and return each apparatus to its appropriate place in the laboratory.
 Module 1: Lesson 3 Assignment
Module 1: Lesson 3 Assignment
If you have not already done so, retrieve your copy of the Module 1: Lesson 3 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment and save it in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
1.17. Page 5
Module 1—Thinking Energy
 Self-Check
Self-Check
SC 5. Calculate the energy required to increase the temperature of a 175-g piece of copper from –25.0ºC to 25.0ºC.
SC 6. 6.0 x 103 J of energy is added to 150 g of water at 25.2ºC. Calculate the final temperature of the water.
SC 7. Calculate the energy released by 1.50 kg of hot water at 100.0ºC as it cools to room temperature (assume 24.5ºC).
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 5.
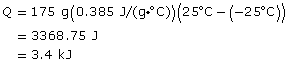
SC 6.
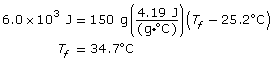
SC 7.
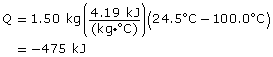
475 kJ are released from the hot water as it cools.
 Reflect and Connect
Reflect and Connect

In this lesson you have investigated specific heat capacity and considered the effect that a difference in the specific heat capacity of water and/or of air may have on the ability of a sleeping bag to function in cold temperatures. The insulating properties of materials, whether used in a sleeping bag or for other purposes, are influenced by specific heat capacity and thermal conductance.
For example, the polystyrene used to make the cups of the calorimeter you used is a significantly better insulator than the glass used to make a beaker. Although polystyrene has a higher specific heat capacity than glass, polystyrene has a significantly lower thermal conductance, making it a better insulating material.
Choice of Sleeping Bag
Even if you don’t plan to have your ecotour participants camp outdoors, a sleeping bag can be helpful if there are unexpected weather changes or if someone is injured. What type of sleeping bag will you provide for your clients?
Use the Internet and other sources to collect information about different types of sleeping bags. You may wish to collect information about comfort rating, design, insulating material, weight, durability, ease of cleaning, cost, and other relevant factors.
RC 2. Using the information you collect, prepare a chart comparing a minimum of two sleeping bag models. Choose which model will best meet your clients' needs and justify your choice. Add your results to your ecotour planning sub-folder.
This is the second of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Reflect on the Big Picture
Reflect on the Big Picture
Can you think of other ecotour aspects in which specific heat capacity might be important? Write these down, and place a copy of your answers in your course folder. You may wish to refer back to this list later in Module 1.
 Going Beyond
Going Beyond

© Franck Boston /shutterstock
Many new homes are being constructed in Alberta, and a large number of these buildings have highly energy-efficient designs. Use the Internet to find out more about the term R-2000. Find standards for new home construction, and compare these standards to construction practices of ten or more years ago. For example, you may wish to compare the design and materials used in your own home to those that meet R-2000 standards. How important is insulation and the specific heat capacity of materials to R-2000 design?
 Module 1: Lesson 3 Assignment
Module 1: Lesson 3 Assignment
Submit your completed Module 1: Lesson 3 Assignment to your teacher.
1.18. Page 6
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 3 you considered the following question:
- How can knowledge of specific heat capacity be used in the design of a sleeping bag?
You learned about specific heat capacity. Many devices use knowledge of the laws of thermodynamics and specific heat capacity in their designs. You also completed an investigation in which you used experimental data to calculate the specific heat capacity for water and to evaluate a calorimeter.
In future lessons you will continue to apply your knowledge about calorimetry and energy transfers to investigate energy changes in chemical systems.
Lesson Glossary
magnitude: a value that indicates size or quantity
quantitative: an observation that uses a numeric value
specific heat capacity: a physical property of matter
It is the energy required to raise the temperature of one gram of a substance one degree Celsius.
thermal conductance: the rate at which thermal energy flows through a substance
1.19. Lesson 4
Module 1—Thinking Energy
Lesson 4—Enthalpy Changes
 Get Focused
Get Focused

© Courtesy of Shauna Maria Whyte
Cross-country skiing is a popular sport and may be one of the activities participants in your ecotour will undertake. Skiing is physically strenuous and requires a lot of energy. In addition to the large amount of energy used to cross the snow-covered terrain, a skier will expend energy to maintain his or her body temperature in cold winter weather. To conserve energy, paralympians sometimes use hot packs on their legs while training and racing on extremely cold days. Can you think of a similarity between a human body and a hot pack with respect to energy changes?

In previous science courses you learned that the human body is designed to convert chemical energy from the food we eat into energy cells can use. Heat is a common by-product of cellular processes. A hot pack also releases energy as heat when a change occurs in the matter inside. Energy changes can be classified as either exothermic or endothermic. An exothermic process will warm its surroundings as it radiates energy. Endothermic processes absorb heat from the surroundings, often reducing the temperature of the surroundings.
exothermic: a process that radiates energy to its surroundings
endothermic: a process that absorbs energy from its surroundings
From your study of biology you know that humans are made up of millions of tiny cells. When you study chemistry you also consider the interactions of matter at the particle level. Why do the reactions involved in the use of food and those involved in the hot pack create heat? What is happening in both of these processes at the particle level, and how do those changes relate to the energy change involved in each of these processes? In Lesson 4 you will explore enthalpy changes.
Consider the following questions as you complete Lesson 4:
- What changes occur during a chemical reaction at the particle level?
- Why do chemical changes and energy changes occur at the same time?
What is enthalpy?
 Module 1: Lesson 4 Assignment
Module 1: Lesson 4 Assignment
In this lesson you will
- draw cartoons to illustrate the different kinds of energy matter can have
- analyze calorimetric data and quantify the energy changes that occur
- review the assumptions involved in calorimetry as they relate to enthalpy changes
There is no assignment for this lesson; however, you will complete Try This, Reflect and Connect, and Reflect on the Big Picture activities, some of which may be useful for completing your Module 1 Assessment. You will also receive further instructions on, and you will complete, part of the Module 1 Assessment: Recharging Hot Packs.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.20. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
Kinetic Molecular Theory
kinetic molecular theory: a theory that states that small particles that make up a substance are in continuous motion and collide with each other and with objects in their path
kinetic energy: a form of energy related to the motion of particles
Kinetic energy can involve vibration, rotation, or translation within or of a particle. Kinetic energy changes are often observed as a temperature change in matter.
chemical potential energy: a stored form of energy dependent on the relative positions of particles
All matter is made of tiny particles. The kinetic molecular theory states that small particles that make up a substance are in continuous motion and collide with each other and with objects in their path. Motion associated with particles can involve more than collisions.
Read the bulleted lists under “Heat Transfer and Enthalpy Change” at the bottom of page 487 in the textbook to review the kinds of motion that are associated with a particle’s kinetic energy and the associations between particles that involve chemical potential energy.
 Try This
Try This
Chemical Potential and Kinetic Energy
TR 1. By hand or digitally, prepare cartoons that depict the different kinds of energy matter can have. Save your work in your course folder and submit a copy to your teacher.
 Read
Read
The sum of the kinetic and potential energies in a system is called enthalpy. A system’s enthalpy cannot be measured directly but can be determined indirectly by comparing the change in enthalpy of the products relative to the reactants.
In Lesson 3 you used a calorimeter to measure energy changes that were the result of changes involving matter. How does the energy change for a process measured using a calorimeter relate to an enthalpy change?
Read page 488 in the textbook to learn more.
 Try This
Try This
Energy Measured in a Calorimeter
TR 2. Write a paragraph to respond to the question in the Read activity above: how does the energy change for a process measured using a calorimeter relate to an enthalpy change? Save your response in your course folder and submit a copy to your teacher.
TR 3. Can you recall any other assumptions that are made in the design of a calorimeter or in interpreting the data collected from the calorimeter? How do these assumptions make it possible to measure the enthalpy change of a process that occurs within a calorimeter? Make sure you save a copy of your answers to these questions in your course folder as they will help you complete the next activity.
 Read
Read
Read page 489 and work through “Sample problem 11.1” and “Communication example 2” in the textbook.
1.21. Page 3
Module 1—Thinking Energy
 Reflect and Connect
Reflect and Connect
Energy Change of a Hot Pack
In this lesson you learned about enthalpy change and how an enthalpy change might be quantified using calorimetry. Complete the following activity to test your understanding of what you have learned.
The following data were collected from a calorimetry experiment investigating the energy released by the hot pack shown in the video. Use your observations from watching the video and the data provided to complete the Analysis section below.
Data
mass of water in calorimeter = 550 g
initial temperature of water = 22.3ºC
highest temperature of water = 30.7ºC
Analysis
RC 1. The contents of the hot pack are listed as sodium acetate, water, and a metal disk. State the change that occurs to the arrangement of the sodium acetate particles during the operation of the hot pack.
RC 2. Is the type of change identified in your answer to RC 1 an example of a change in kinetic energy or potential energy? Support your answer.
RC 3. Explain why the change identified in your answer to RC 2 represents an enthalpy change.
RC 4. Use the data provided to determine the enthalpy change of the hot pack.
RC 5. Is the enthalpy change of the hot pack an exothermic or endothermic process? Support your answer by making specific reference to the data provided.
Save a copy of your responses in your course folder and submit a copy to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture
Recharging Hot Packs

The hot pack used in the previous activity is reusable. When the hot pack is placed in boiling water, the solid sodium acetate dissolves and forms a colourless solution within the bag. If your ecotour takes place in winter, hot packs may be an essential piece of equipment for your operation. That could mean a lot of hot packs.
RBP 1. How might a reusable hot pack containing sodium acetate help minimize the negative ecological impact of your ecotour operation? Consider aspects such as energy changes and the reactivity and toxicity of the substances associated with the hot pack in developing your answer. Place a copy of your response in your ecotour planning sub-folder.
This is the third of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Module 1: Lesson 4 Assignment
Module 1: Lesson 4 Assignment
There is no assignment for this lesson.
1.22. Page 4
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 4 you considered the following questions:
- What changes occur during a chemical reaction at the particle level?
- Why do chemical changes and energy changes occur at the same time?
- What is enthalpy?
You studied both theoretical and empirical aspects of matter and energy changes. You used cartoons and other methods to explain changes in matter and you analyzed data to calculate energy changes. You learned that an enthalpy change is the change in the kinetic and potential energy of a system and that an enthalpy change can be quantified using a calorimeter.
Lesson Glossary
chemical potential energy: a stored form of energy dependent on the relative positions of particles
endothermic: a process that absorbs energy from its surroundings
enthalpy: the total kinetic and potential energy in a system
exothermic: a process that radiates energy to its surroundings
kinetic energy: a form of energy related to the motion of particles
Kinetic energy can involve vibration, rotation, or translation within or of a particle. Kinetic energy changes are often observed as a temperature change in matter.
kinetic molecular theory: a theory that states that small particles that make up a substance are in continuous motion and collide with each other and objects in their path
1.23. Lesson 5
Module 1—Thinking Energy
Lesson 5—Molar Enthalpy
 Get Focused
Get Focused

Historically, Inuit living in the Arctic depended primarily on seal oil prepared from blubber as a fuel source for light, cooking, and heating. While using seal oil may seem peculiar, it was an ingenious use of this resource because of the oil’s high caloric content, availability, and ease of use.
In most modern societies, factors beyond availability and ease of use must be considered when choosing fuels. Cost and transportation are important factors. However, perhaps the most important consideration is energy richness.
How would you define the energy richness of a fuel? Could you incorporate some of the concepts you learned about in earlier Module 1 lessons to define energy richness? How might you determine the energy richness of a fuel? In this lesson you will learn how to use enthalpy changes to assess fuels.
Consider the following question as you complete Lesson 5:
-
How can the energy richness of different fuels be compared?
 Module 1: Lesson 5 Assignment
Module 1: Lesson 5 Assignment
Download a copy of the Module 1: Lesson 5 Assignment to your computer now. You will receive further instructions on how to complete this assignment later in this lesson.
In this lesson, you will also receive further instructions on and you will complete part of the Module 1 Assessment: Evaluating Different Fuels.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.24. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
In Lesson 4 you learned about enthalpy change. An enthalpy change occurs in every chemical reaction due to the change in potential energy associated with the change in bonding of the atoms involved. The energy change associated with a chemical reaction is called the enthalpy of reaction.
It may not surprise you that overall energy changes associated with a reaction depend on the quantity of matter involved. For example, to get more heat out of your campfire for your ecotour participants, you would put more wood, or fuel, on the fire. It might surprise you that enthalpy change is not the same for all combustion reactions. You may have encountered this when you noticed that some fuels appear to release more energy than others when they combust.
How do you compare the enthalpy of reaction for different reactions? Read page 490 and work through “Sample problem 11.2” and “Communication example 3” on page 491 in the textbook to find out.
 Try This
Try This
Molar Enthalpy of Fuel Combustion
Later in this lesson you will be asked to compare fuels. You will be given information about the molar enthalpy of reaction for each of the fuels to be compared. What does this information mean? Can you guess how a molar enthalpy of reaction for each fuel was determined?
TR 1. State the purpose of determining a molar enthalpy of combustion for different fuels.
TR 2. Describe an experiment that could be used to determine the molar enthalpy of combustion for a fuel. You may wish to determine the values you would need to measure during your experiment and then draft a formula that uses all your measured values.
Read the bottom section of page 491 in the textbook for help completing your description. You may wish to share your response to questions TR 1 and TR 2 with your teacher.
 Read
Read
The enthalpy of reaction is the change in enthalpy of a chemical system. Molar enthalpy is a change in enthalpy of a chemical system per mole. Molar enthalpy is helpful when assessing different fuels—just as “apples to apples” are compared in everyday life, molar enthalpy allows comparing “moles to moles” in chemistry.
Work through “Communication example 4” on page 492 in the textbook.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 9–13 on page 492 in the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 9.
a. ΔfH
b. ΔdH
c. ΔfHm
d. ΔdHm
Practice 10.
Similarities |
Differences |
Both contain the Δ and H symbols. |
In molar enthalpy, the final character is a subscript lower-case m, indicating molar. |
Both represent the energy change when a chemical change occurs. |
The molar enthalpy of reaction is for a defined quantity of substance, one mole of a reactant or a product, involved in the reaction being studied. |
Practice 11.
A positive value indicates that the process described is an endothermic process (energy is absorbed by the system when the reaction occurs). A negative value means that the process is exothermic (energy is released by the system when the reaction occurs).
Practice 12.
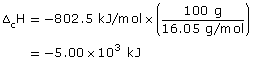
Practice 13.
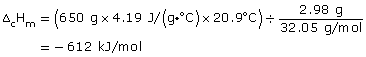
1.25. Page 3
Module 1—Thinking Energy
 Lesson 5 Lab: Molar Enthalpy of Neutralization
Lesson 5 Lab: Molar Enthalpy of Neutralization
Background Information
The molar enthalpy for many chemical reactions can be determined experimentally, provided you have appropriate equipment. Earlier you constructed a calorimeter using nested polystyrene cups. In this investigation you will use a calorimeter to determine the molar enthalpy change for a neutralization reaction between aqueous sodium hydroxide and sulfuric acid solutions.
Purpose
To determine the molar heat of a chemical change (reaction), and to compare the experimentally obtained value with a widely accepted value for the ΔnHm for sodium hydroxide.
Experimental Design
The heat of reaction will be measured by combining 30.0 mL of 1.0 mol/L H2SO4(aq) with 50.0 mL of 1.0 mol/L NaOH(aq). The two reactants will be combined in a simple calorimeter made of two polystyrene cups nested together. Assume that the specific heat capacity of both of these substances is equal to that of water, and that each of these solutions has a density identical to water.
![]() Retrieve your copy of the Module 1: Lesson 5 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 5 Assignment.
Retrieve your copy of the Module 1: Lesson 5 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 5 Assignment.
Materials
- three polystyrene cups
- two 50-mL graduated cylinders
- thermometer
- mol/L H2SO4(aq)
- mol/L NaOH(aq)
If you have access to the materials listed, you may be able to perform this investigation.
![]() If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 5 Lab: Molar Heat of Neutralization.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 5 Lab: Molar Heat of Neutralization.” If you will not be completing the lab youself, remember to record data and observations as you view the presentation.
Procedure
Step 1: Nest two polystyrene cups.
Step 2: Measure 30.0 mL of 1.0 mol/L H2SO4(aq) solution using a 50-mL graduated cylinder and pour it into the calorimeter.
Step 3: Using another 50-mL graduated cylinder, measure 50.0 mL of NaOH(aq) solution.
Step 4: Record the temperature of each solution to the nearest 0.1°C.
Step 5: Pour the NaOH solution into the calorimeter. Use the third cup as a lid. Insert the thermometer into a hole in the lid, and slowly stir the mixture with the thermometer.
Step 6: Record the highest temperature obtained to the nearest 0.1°C.
Step 7: Pour the reaction mixture down the sink. The reaction mixture contains dilute excess sulfuric acid and acqueous sodium sulfate, and it does not require specific treatment prior to disposal. Rinse the calorimeter and other glassware used. Put all equipment away.
 Module 1: Lesson 5 Assignment
Module 1: Lesson 5 Assignment
Retrieve your copy of the Module 1: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete Part 1—Lab: Molar Heat of Neutralization. You will receive information later in the lesson on when to complete the rest of the Lesson 5 Assignment and on when to submit your work to your teacher.
1.26. Page 4
Module 1—Thinking Energy
 Module 1: Lesson 5 Assignment
Module 1: Lesson 5 Assignment
Evaluating Different Fuels

© 2008 Jupiterimages Corporation
How would what you know about molar enthalpy apply to choosing a fuel for your ecotour operation? Are there other aspects to consider in your choice of fuel?
Retrieve your copy of the Module 1: Lesson 5 Assignment that you saved to your computer earlier in this lesson. Complete Part 2—Evaluating Different Fuels. You will receive information later in the lesson on when to submit your work to your teacher.
Place a copy of your answers in your ecotour planning sub-folder. This is the fourth of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Read
Read
Reviews and summaries are an important part of learning. Read the “Summary” section on page 493 of the textbook.
 Try This
Try This
To review what you have learned in the first five lessons in Module 1, try one of the following learning strategies:
- Review the concept map or graphic organizer you have been adding to throughout Module 1. Remember to use linking statements to explain how you understand the concepts to be connected.
- Use the Module 1 Concept Organizer as a starting point for another style of summarixing information (e.g., a list of key words, a concept map, a flow chart, or an outline).
- Review the lesson glossaries by covering up the definitions and defining each term in your own words.
- Create test questions and answers on flash cards.
- Write a script and record an audio file, podcast, or video introducing and explaining the major points listed in the “Summary” section on page 493 of the textbook.
Save your work in your course folder.
 Reflect on the Big Picture
Reflect on the Big Picture
Earlier in this lesson you were asked to prepare a list of similarities and differences between enthalpy change and molar enthalpy. You realized there are many subtle differences to keep in mind as you continue to work through Unit 1.
One way to demonstrate your understanding of a concept is to create an analogy. An analogy explains a concept by comparing it to a similar but more familiar concept. For example, to express how fast people drive on German highways, you might say “Driving on the Autobahn in Germany is like driving on a racetrack.”
RBP 1. Develop an analogy to explain what a molar enthalpy value is or is not. Your analogy may need to be more than one sentence.
 Module 1: Lesson 5 Assignment
Module 1: Lesson 5 Assignment
Submit your completed Module 1: Lesson 5 Assignment and a copy of your analogy to your teacher.
1.27. Page 5
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 5 you considered the following question:
- How can the energy richness of different fuels be compared?
Molar enthalpy of reaction is a value describing the energy change associated with a chemical process per mole of one of the reactants or products involved in the process. In this lesson you calculated a molar enthalpy for a neutralization reaction, and you used molar enthalpy values to compare different fuels per mole of fuel.
As society is becoming more aware of humanity's impact on the environment, people are attempting to ”go green.” Your tour participants will expect your ecotour to use energy-rich alternative fuels.
Lesson Glossary
enthalpy of reaction: the change in the energy of a chemical system as it proceeds from reactants to products
molar enthalpy: a change in enthalpy of a chemical system per mole
1.28. Lesson 6
Module 1—Thinking Energy
Lesson 6—Communicating Enthalpy Change
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
Campfires are a common sight on ecotours. They are a means of cooking food, providing warmth, and inspiring stories. Fire involves a combustion reaction—one of the most common exothermic reactions.
As a science student you have been taught to observe chemical reactions for evidence of change, including energy change. What kinds of energy changes are occurring during a combustion reaction? In some reactions you can feel an increase in temperature or see light emitted.
In previous lessons you learned to quantify the energy change, as heat, associated with a chemical reaction. In this lesson you will further expand your understanding of enthalpy change. You will investigate how each of the following methods allows a process' enthalpy change to be clearly identified and associated with a specific chemical reaction:
- molar enthalpy of reaction for a specific reactant
- enthalpy change for a balanced reaction equation
- inclusion of the energy as a term in a balanced reaction equation
- chemical potential energy diagrams
Consider the following question as you complete Lesson 6:
- How are enthalpy changes communicated?
 Module 1: Lesson 6 Assignment
Module 1: Lesson 6 Assignment
There is no assignment for this lesson. However, you will be asked to submit samples of your work to your teacher for feedback where instructed.
In Lesson 8 you will complete an assignment on material covered in Lessons 6, 7, and 8. In preparation for the Lesson 8 Assignment, you may wish to summarize what you learn in Lessons 6, 7, and 8 as you proceed through these lessons. Your summary may take one of many forms (e.g., list, outline, concept map), depending on your individual learning style. Save a copy of your summary in your course folder so that you may quickly add to and review your summary in preparation for the Lesson 8 Assignment.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.29. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
In Lesson 5, you learned to calculate the enthalpy of a reaction per mole of one of the process' reactants or products. Communicating a reaction enthalpy in this way allows specific information to be expressed.
Enthalpy changes are communicated in four ways:
- molar enthalpy of reaction for a specific reactant
- enthalpy change for a balanced reaction equation
- inclusion of the energy as a term in a balanced reaction equation
- chemical potential energy diagrams
Read the text and work through the “Sample problems” and the “Communication examples” on pages 495–499 in the textbook. Complete TR 1 as you read.
 Try This
Try This
TR 1. Complete a table like the following in your course folder to summarize the four methods as you read about them:
Question |
Molar enthalpy of reaction for a specific reactant |
Enthalpy change for a balanced reaction equation | Inclusion of the energy as a term in a balanced reaction equation | Chemical potential energy diagrams |
How is this method done? |
||||
What do I need to remember to do this method correctly? |
TR 2. Test your understanding and your summaries by attempting “Communication example 4” on page 500 in the textbook.
![]() Post your table in the discussion area for your class and read the summaries of at least two other students. If necessary, revise your table. Save a copy of your revised table in your course folder.
Post your table in the discussion area for your class and read the summaries of at least two other students. If necessary, revise your table. Save a copy of your revised table in your course folder.
 Self-Check
Self-Check
SC 1. Complete “Section 11.3” questions 1, 2, 3, 4, 5, and 7 on page 501 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Section 11.3 1.
- The symbols used represent the following: Δ = change, c = combustion, H = enthalpy, m = molar, º = standard conditions. Therefore, the symbols represent the molar enthalpy change for a combustion reaction (e.g., methane at standard conditions).
- The symbols used represent the following: n = amount, Δ = change, H = enthalpy, m = molar, º = standard conditions. Therefore, the symbols represent the moles of one substance (e.g., propane) involved in a chemical process multiplied by its molar enthalpy of reaction (e.g., molar heat of combustion for propane).
- The symbol used represents the thermal energy change for water.
Section 11.3 2.
- ΔrHmº = +227.4 kJ/mol
2 C(s) + H2(g) → C2H2(g) ΔrHº = +227.4 kJ
2 C(s) + H2(g) + 227.4 kJ → C2H2(g)
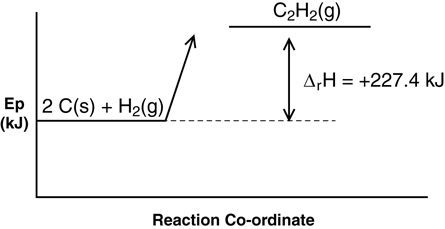
- ΔrHmº = +1675.7 kJ/mol
Al2O3(s) → 2 Al(s) + 3/2 O2(g) ΔrHº = +1675.7 kJ
Al2O3(s) + 1675.7 kJ → 2 Al(s) + 3/2 O2(g)
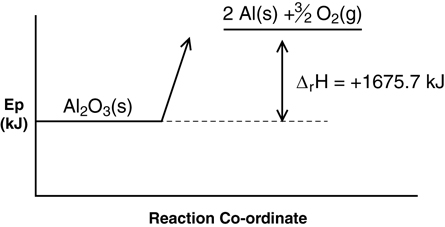
- ΔrHmº = -393.5 kJ/mol
C(s) + O2(g) → CO2(g) ΔrHº = -393.5 kJ
C(s) + O2(g) → CO2(g) + 393.5 kJ
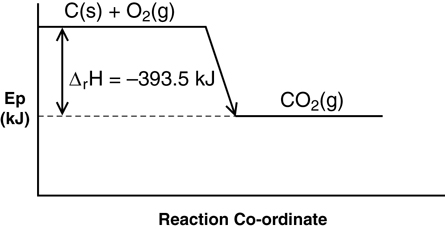
Section 11.3 3.
- ΔrHmº = -241.8 kJ/mol of hydrogen
- ΔrHmº = -318.0 kJ/mol of ammonia
- ΔrHmº = +81.6 kJ/mol of nitrogen
- ΔrHmº = -372.8 kJ/mol of iron
Section 11.3 4.
- ΔrH = -114 kJ
- H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l) ΔrH = -114 kJ
- ΔrH = -114 kJ / 1 mol = -114 kJ/mol of sulfuric acid
- ΔrH = -114 kJ / 2 mol = -57 kJ/mol of aqueous sodium hydroxide
Section 11.3 5.
- CH3OH(l) + 3/2 O2(g) → CO2(g) + 2 H2O(g) + ΔcH = -725.9 kJ
- C(s) + ¼ S8(s) → CS2(l) ΔfH = +89.0 kJ
- ZnS(s) + 3/2 O2(g) → ZnO(s) + SO2(g) ΔcH = -441.3 kJ
- Fe2O3(s) → 2 Fe(s) + 3/2 O2(g) ΔsdH = +824.2 kJ
Section 11.3 7.
- H2(g) + ½ O2(g) → H2O(g) ΔrH = -285.8 kJ
H2O(g) → H2(g) + ½ O2(g) ΔrH = +285.8 kJ
- The magnitude of the enthalpy changes are identical, except that the combustion reaction is exothermic and the decomposition reaction is endothermic.
ΔrH(combustion) = -ΔrH(decomposition)
1.30. Page 3
Module 1—Thinking Energy
 Reflect on the Big Picture
Reflect on the Big Picture
Evaluating Two Liquid Fuels
Liquid fuels might be very useful in the operation of your ecotour. Two popular choices are methanol and ethanol.
RBP 1. Analyze the information below. Explain how your understanding of communicating enthalpy changes could help you identify which of these fuels is preferred with respect to its energy of combustion.
Fuel |
Combustion Reaction Equation |
ΔcHº |
Methanol |
2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(g) |
-1275.8 kJ |
Ethanol |
C2H5OH(l) + 3 O2(g) → 2 CO2(g) + 3 H2O(g) |
-1234.8 kJ |
Save a copy of your response in your ecotour planning sub-folder. This is the fifth of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Module 1: Lesson 6 Assignment
Module 1: Lesson 6 Assignment
There is no assignment for this lesson. Make sure you have saved a summary of what you've learned in Lesson 6 in your course folder. You will need this summary to complete the Lesson 8 Assignment.
1.31. Page 4
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 6 you considered the following question:
- How are enthalpy changes communicated?
You learned that enthalpy changes can be communicated in the following four ways:
- molar enthalpy of reaction for a specific reactant
- enthalpy change for a balanced reaction equation
- inclusion of the energy as a term in a balanced reaction equation
- chemical potential energy diagrams
Each of these methods is useful for expressing the type and magnitude of enthalpy changes. You evaluated the importance of expressing enthalpy changes in each of these ways. You explained how communicating enthalpy changes could help you identify which fuels might be preferred for your ecotour.
1.32. Lesson 7
Module 1—Thinking Energy
Lesson 7—Hess’ Law
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
Hiking is a popular ecotour activity. Participants often want to see remote, untouched areas to get a sense of the natural habitat. Hiking in the Drumheller Badlands provides a great opportunity to learn about geology, paleontology, and other science disciplines while enjoying hiking's many other benefits.
In the photograph shown, the trail the hikers are on dips downward and then slopes upward again. How do these small changes in elevation affect the hikers’ level of effort?
In the last lesson you used chemical potential energy diagrams to represent the enthalpy change of a reaction. What does a change in position on a potential energy diagram represent?
You have probably hiked over hilly terrain before. Did you break your hike into manageable goals or steps? Did breaking your hike into steps affect the outcome of your day’s effort? Chances are that thinking in steps and taking frequent breaks got you to the end of your journey refreshed and having enjoyed some of the sights along the way.
Breaking a hike into small steps could be an analogy for a chemical reaction performed as a series of steps that results in the desired products. Is there a difference in the enthalpy change of reaction for the total process when it occurs in steps?
Consider the following questions as you complete Lesson 7:
- What is Hess’ law?
- How does Hess’ law relate to enthalpies of reaction?
 Module 1: Lesson 7 Assignment
Module 1: Lesson 7 Assignment
Download a copy of the Module 1: Lesson 7 Assignment to your computer now.
In Lesson 8 you will complete an assignment that covers material from Lessons 6, 7, and 8. It is important that you continue to summarize what you learn as you work through these lessons.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.33. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
In Lesson 2 you used a calorimeter to measure the enthalpy of a reaction. You also learned that by using a bomb calorimeter you can improve the reliability and range of reactions that you can investigate. However, is it possible to measure the enthalpy change of every reaction using a calorimeter? Is it possible to use your understanding of chemical potential energy to explain why energy changes occur during chemical reactions? Have you considered how this understanding might be used to determine the enthalpy change for a process if it cannot be done experimentally?
Read pages 502–503 in the textbook to learn about Hess’ law.
 Self-Check
Self-Check
SC 1. Identify two instances in which it is difficult to determine the energy change for a reaction experimentally using equipment normally found in a high school laboratory.
SC 2. Sketch “Figure 2” on page 503 of the textbook. Add details to your sketch to
- identify the separate reactions (steps) in the process
- explain why the overall enthalpy change is only -110.5 kJ, not -393.5 kJ
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. It may not be possible to determine the energy change for a reaction experimentally if the reaction occurs too slowly, if equipment is unavailable, or if there are safety concerns.
SC 2.
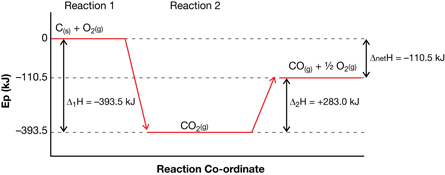
The overall change is -110.5 kJ, which represents the difference between the initial and final potential energies of the reactants and the final product. The sum of the reaction enthalpies is -110.5 kJ.
 Try This
Try This
Use your answers to the Self-Check questions and the information in your textbook to demonstrate your understanding of Hess' law by answering the following questions.
Consider the following information about a theoretical chemical system:
Reaction 1: AB → A + B ΔrH = +75 kJ
Reaction 2: A + CD → AC + D ΔrH = -50 kJ
Reaction 3: BD → B + D ΔrH = -125 kJ
Net Reaction: AB + CD → AC + BD
TR 1. Demonstrate how reactions 1–3 can be combined to yield the net reaction.
TR 2. Demonstrate how reactions 1–3 can be depicted on a potential energy diagram such that the enthalpy change for the net reaction is depicted.
TR 3. Demonstrate how the enthalpy change for reactions 1–3 are combined to result in an enthalpy change for the net reaction.
Submit your responses to your teacher and save a copy in your course folder. If your teacher provides feedback, carefully review his or her feedback and revise your answer, if necessary.
![]() Post your responses to TR 1–3 in the discussion area for your class. Review the responses of at least two other students. Identify where your responses were similar to and different from your classmates' responses. If possible, evaluate the different approaches used by your classmates, and determine whether you might use parts of their strategies. If necessary, revise your answers to TR 1–3 and save a copy of your revised responses in your course folder.
Post your responses to TR 1–3 in the discussion area for your class. Review the responses of at least two other students. Identify where your responses were similar to and different from your classmates' responses. If possible, evaluate the different approaches used by your classmates, and determine whether you might use parts of their strategies. If necessary, revise your answers to TR 1–3 and save a copy of your revised responses in your course folder.
 Read
Read
Work through “Sample problem 11.4” and “Communication example” on pages 503–504 in the textbook.
![]() You may go to the website for your textbook to listen to the explanation of “Sample problem 11.4.” Contact your teacher for instructions on how to access this website.
You may go to the website for your textbook to listen to the explanation of “Sample problem 11.4.” Contact your teacher for instructions on how to access this website.
Review the “Summary” on page 504 of the textbook for clarification of the process used to solve these problems.
 Self-Check
Self-Check
SC 3. Complete “Practice” questions 1–4 on pages 504–505 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Practice 1.
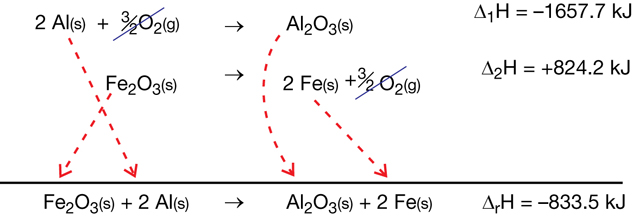
Practice 2.
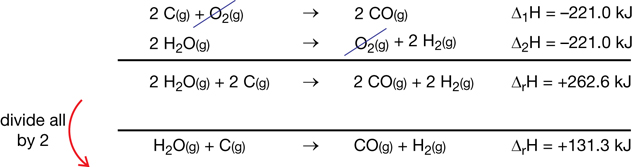
Practice 3.
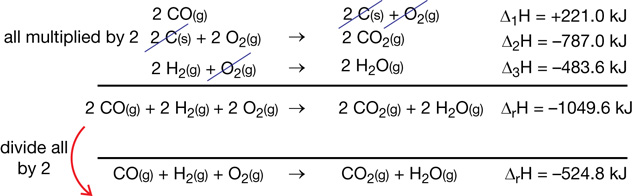
Practice 4.
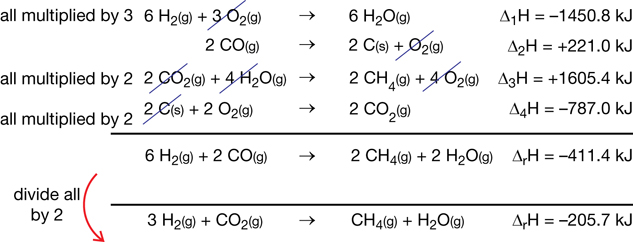
 Try This
Try This
To some people, the most important rules for Hess’ law are as follows:
- If the chemical reaction is reversed, then the sign of the change in enthalpy is reversed.
- If the coefficients of the chemical reaction are altered, then the change in enthalpy is also altered by the same factor.
It is easy to memorize these rules, but it is important to do more than memorize them—you must understand them.
TR 4. Write explanations that identify the scientific principles associated with each of these rules and state why these rules need to be considered when using Hess’ law.
Save a copy of your response in your course folder.
![]() Post a copy of your response to the discussion area for your class. Read the responses of at least two other students. Provide feedback to your classmates to help them ensure their explanations are clear and thorough. Revise your explanations based on the feedback you receive. Save a copy of your revised explanations in your course folder.
Post a copy of your response to the discussion area for your class. Read the responses of at least two other students. Provide feedback to your classmates to help them ensure their explanations are clear and thorough. Revise your explanations based on the feedback you receive. Save a copy of your revised explanations in your course folder.
1.34. Page 3
Module 1—Thinking Energy
 Module 1: Lesson 7 Lab—Applying Hess’ Law
Module 1: Lesson 7 Lab—Applying Hess’ Law
Purpose
To use Hess’ law to determine the molar enthalpy of combustion for a reaction that cannot be determined experimentally with available equipment.
Problem
What is the molar enthalpy of combustion for magnesium?
Prediction
The accepted value for this reaction is –601.6 kJ/mol of magnesium reacted.
![]() Retrieve your copy of the Module 1: Lesson 7 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 7 Assignment.
Retrieve your copy of the Module 1: Lesson 7 Assignment that you saved to your computer earlier in this lesson. You will record data and complete your analysis in your Lesson 7 Assignment.
Materials
- lab apron
- eye protection
- magnesium ribbon
- magnesium oxide powder
- mol/L hydrochloric acid (100.0 mL for each trial)
- three polystyrene cups
- thermometer
- 100-mL graduated cylinder
- scoopula and weighing boat or paper
- steel wool
- electronic balance
- ruler
If you have access to the materials listed, you may be able to perform this investigation.
![]() If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 7 Lab: Applying Hess’ Law.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
If you do not have access to these materials and a supervised laboratory, or if you would like to see the lab performed before you attempt it, view the virtual investigation “Lesson 7 Lab: Applying Hess’ Law.” If you will not be completing the lab yourself, remember to record data and observations as you view the presentation.
Procedure
Part A
Step 1: Construct a calorimeter by nesting two polystyrene cups.
Step 2: Measure 100.0 mL of HCl(aq) and pour it into the calorimeter.
Step 3: Record the temperature of the contents of the calorimeter.
Step 4: Use the ruler to measure approximately 15 cm of magnesium ribbon.
Step 5: Use the steel wool to remove the coating from the magnesium ribbon.
Step 6: Measure and record the mass of the magnesium ribbon using the electronic balance.
Step 7: Add the magnesium ribbon to the calorimeter, and gently stir the calorimeter contents using the thermometer.
Step 8: Place the lid on the calorimeter, and record the highest temperature of the calorimeter contents.
Step 9: Empty the calorimeter into the appropriate waste container, and rinse the calorimeter with water.
Part B
Step 1: Measure 100.0 mL of HCl(aq) and pour it into the calorimeter.
Step 2: Record the temperature of the contents of the calorimeter.
Step 3: Measure and record approximately one gram of magnesium oxide.
Step 4: Add the magnesium oxide to the calorimeter, and gently stir the calorimeter contents using the thermometer.
Step 5: Place the lid on the calorimeter, and record the highest temperature of the calorimeter contents.
Step 6: Empty the calorimeter into the appropriate waste container and rinse the calorimeter with water.
Step 7: Return all materials to their place in the lab.
 Module 1: Lesson 7 Assignment
Module 1: Lesson 7 Assignment
If you have not already done so, retrieve your copy of the Module 1: Lesson 7 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment and save it in your course folder. Submit a copy of your completed Module 1: Lesson 7 Assignment to your teacher.
Make sure you have saved a summary of what you’ve learned in Lesson 7 in your course folder. You will need this summary to complete the Lesson 8 Assignment.
1.35. Page 4
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 7 you considered the following questions:
- What is Hess’ law?
- How does Hess’ law relate to enthalpies of reaction?
Hess’ law is a thermodynamic principle that states that the addition of chemical equations yields a net chemical equation with an enthalpy change equal to the sum of the enthalpy changes of the steps. Hess’ law can be used to determine the enthalpy change for reactions that are not able to be investigated experimentally. You evaluated the process used to investigate chemical reactions as multistep processes, and you completed a laboratory exercise to test a hypothesis made using Hess’ law.
1.36. Lesson 8
Module 1—Thinking Energy
Lesson 8—Molar Enthalpies of Formation
 Get Focused
Get Focused

© 2008 Jupiterimages Corporation
Since ecotours attract people from all over the world, you may wish to employ someone with a second language for your ecotour. Communication with your ecotour participants is very important, especially where activities involve important safety instructions.
In previous lessons you learned many new terms, symbols, and methods for communicating aspects of thermodynamics. In Lesson 8 you will further your study of the enthalpy changes in chemical reactions by investigating the use of molar enthalpies of formation.
Consider the following question as you complete Lesson 8:
- How are standard molar enthalpies of formation used to determine the enthalpy change associated with chemical reactions?
 Module 1: Lesson 8 Assignment
Module 1: Lesson 8 Assignment
Download a copy of the Module 1: Lesson 8 Assignment to your computer now. This assignment covers material learned in Lessons 6,7, and 8.
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.
1.37. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
reference energy state: a reference point at which the potential energy of the elements in their most stable form at SATP is defined as zero
standard enthalpy of formation: the enthalpy change calculated from the measurements of a formation reaction under standard conditions
In previous lessons you may have noticed the difference between the symbols ΔrHº and ΔrH. The degree symbol means that a reaction enthalpy or other property of a system was measured at a standard state. A standard state is a defined set of conditions. You may recall learning two sets of standard conditions when working with gases: STP and SATP.
Read the first two paragraphs on page 510 in the textbook to learn more about the standard used in enthalpy determinations. Take note of the definitions for reference energy state and standard enthalpy of formation.
 Self-Check
Self-Check
SC 1. Using an energy diagram, explain how defining elements as having a standard enthalpy of formation allows for the determination of standard enthalpies of formation for compounds.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. 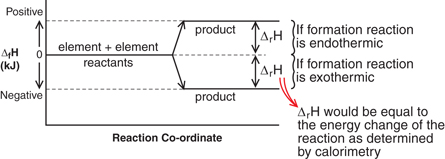
 Read
Read
thermal stability: the tendency of a compound to resist decomposition when heated
The lower a compound's standard enthalpy of formation, the more stable the compound.
Read the remainder of page 510 in the textbook to learn about the concept of thermal stability. Keep an image of an energy diagram fresh in your mind as you read.
Does the analogy stated in the textbook make sense to you? Which substance has greater thermal stability: octane (ΔfHmº = −250.1 kJ/mol) or trinitrotoluene (TNT, ΔfHmº = +123.6 kJ/mol)? Is your conclusion consistent with the reputations of these two compounds?
 Read
Read
Work through “Sample problem 11.5” on page 511 of the textbook to see how molar enthalpies of formation can be used to calculate enthalpies of reaction. You may also wish to read pages 512–513.
 Self-Check
Self-Check
SC 2. Complete “Section 11.5” questions 2 and 3 on page 514 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 2.
Section 11.5 2.
-
Reactants
Products
Species
CH4(g)
H2O(g)
CO(g)
H2(g)
Coefficient (mol)
1
1
1
3
ΔfHmº (kJ/mol)
−74.6
−241.8
−110.5
0
Σ n ΔfHmº
= [(1 mol × −74.6 kJ/mol)
+ (1 mol × −241.8 kJ/mol)]
= −316.4 kJ= [(1 mol × −110.5 kJ/mol)
+ (3 mol × 0 kJ/mol)]
= −110.5 kJΔrHº
= −110.5 kJ − (−316.4 kJ)
= +205.9 kJ
Reactants
Products
Species
CO(g)
H2O(g)
CO2(g)
H2(g)
Coefficient (mol)
1
1
1
1
ΔfHmº (kJ/mol)
−110.5
−241.8
−393.5 kJ
0
Σ n ΔfHmº
= [(1 mol × −110.5 kJ/mol)
+ (1 mol × −241.8 kJ/mol)]
= −352.3 kJ= [(1 mol × -393.5 kJ/mol)
+ (1 mol × 0 kJ/mol)]
= −393.5 kJΔrHº
= −393.5 kJ − (−352.3 kJ)
= −41.2 kJ
Reactants
Products
Species
N2(g)
H2(g)
NH3(g)
Coefficient (mol)
1
3
2
ΔfHmº (kJ/mol)
0
0
−45.9
Σ n ΔfHmº
= [(1 mol × 0 kJ/mol)
+ (3 mol × 0 kJ/mol)]
= 0 kJ= (2 mol × −45.9 kJ/mol)
= −91.8 kJΔrHº
= −91.8 kJ − (0 kJ)
= −91.8 kJ
Reactants
Products
Species
NH3(g)
O2(g)
NO(g)
H2O(g)
Coefficient (mol)
4
5
4
6
ΔfHmº (kJ/mol)
−45.9
0
+91.3
−241.8
Σ n ΔfHmº
= [(4 mol × −45.9 kJ/mol)
+ (5 mol × 0 kJ/mol)]
= −183.6 kJ= [(4 mol × +91.3 kJ/mol)
+ (6 mol × −241.8 kJ/mol)]
= −1085.6 kJΔrHº
= −1085.6 kJ − (−183.6 kJ)
= −902.0 kJ
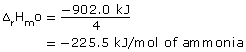
Reactants
Products
Species
NO(g)
O2(g)
NO2(g)
Coefficient (mol)
2
1
2
ΔfHmº (kJ/mol)
+91.3
0
+33.2
Σ n ΔfHmº
= [(2 mol × +91.3 kJ/mol)
+ (1 mol × 0 kJ/mol)]
= +181.6 kJ= (2 mol × +33.2 kJ/mol)
= +66.4 kJΔrHº
= +66.4 kJ − (+181.6 kJ)
= −116.2 kJ
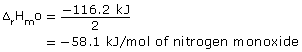
Reactants
Products
Species
NO2(g)
H2O(g)
HNO3(l)
NO(g)
Coefficient (mol)
3
1
2
1
ΔfHmº (kJ/mol)
+33.2
−285.8
−174.1
+91.3
Σ n ΔfHmº
= [(3 mol × +33.2kJ/mol)
+ (1 mol × −285.8 kJ/mol)]
= −186.2 kJ= [(2 mol × −174.1 kJ/mol)
+ (1 mol × +91.3 kJ/mol)]
= −256.9 kJΔrHº
= −256.9 kJ − (−186.2 kJ)
= −70.7 kJ

 Self-Check
Self-Check
In the previous Self-Check activity you used the standard molar enthalpies of formation for compounds to predict the reaction enthalpy. Can this process be reversed?
If given a reaction enthalpy, most likely determined by experiment, a standard molar enthalpy of formation for a species in the reaction can be determined as long as the standard molar enthalpies of formation for the other species are known.
SC 3. Calculate the standard molar enthalpy of formation for hexane, C6H14(l). Assume that hexane’s standard molar enthalpy of combustion is −4162.9 kJ/mol.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
Balanced Reaction Equation
C6H14(l) + 19/2 O2(g) → 6 CO2(g) + 7 H2O(g) ΔrHmº = –4162.9 kJ/mol C6H14(l)
ΔrHº = –4162.9 kJ/mol x 1 mol C6H14(l)
= –4162.9 kJ
|
Reactants |
Products |
ΔrHº |
||
Species |
C6H14(l) |
O2(g |
CO2(g) |
H2O(g) |
|
Coefficient (mol) |
1 |
19/2 |
6 |
7 |
|
ΔfHmº (kJ/mol) |
? |
0 |
–393.5 |
–241.8 |
|
Σ n ΔfHmº |
= [(1 mol × ΔfHmº(hexane)) + (19/2 mol × 0 kJ/mol)] |
= [(6 mol × −393.5 kJ/mol) + (7 mol × −241.8 kJ/mol)] |
−4162.9 kJ |
||
−4053.6 kJ − (1 mol × ΔfHmº(hexane)) = −4162.9 kJ
ΔfHmº(hexane) = +109.3 kJ/mol
The standard molar enthalpy of formation for hexane is +109.3 kJ/mol.
 Reflect on the Big Picture
Reflect on the Big Picture
Standard molar enthalpies of formation allow for quick predictions of reaction enthalpies. Using the hiking analogy introduced in Lesson 7, these values provide an estimate of the chemical potential energy of each compound in the reaction equation. The difference between the potential energies of the products and the reactants is the reaction enthalpy. Using standard molar enthalpies of formation is a lot like knowing the checkpoints you must reach along your hike.
RBP 1. How would you use information on standard molar enthalpies of formation in your ecotour planning? Identify a situation in which this method of determining reaction enthalpy could be used. Identify where other methods and techniques learned in Module 1 could be applied to the operation of your ecotour.
Save a copy of your response in your ecotour planning sub-folder. This is the last of six assignments that you can submit as part of your Module 1 Assessment. For more information refer to the Module 1 Assessment.
 Module 1: Lesson 8 Assignment
Module 1: Lesson 8 Assignment
Retrieve your copy of the Module 1: Lesson 8 Assignment that you saved to your computer earlier in this lesson. Complete the Assignment. Save a copy of your completed Assignment in your course folder and submit a copy to your teacher.
1.38. Page 3
Module 1—Thinking Energy
 Lesson Summary
Lesson Summary
In Lesson 8 you considered the following question:
- How are standard molar enthalpies of formation used to determine the enthalpy change associated with chemical reactions?
You learned about standards and reference points used to study energy changes. The reference point for standard molar enthalpies of formation states that the potential energy of elements in their most stable form at SATP is 0 kJ/mol. You also learned how to interpret values for standard molar enthalpies of formation to assess the thermal stability of compounds. You used these values much in the same way you used Hess’ law, to calculate a reaction enthalpy.
Lesson Glossary
reference energy state: a reference point at which the potential energy of the elements in their most stable form at SATP is defined as zero
standard enthalpy of formation: the enthalpy change calculated from the measurements of a formation reaction under standard conditions
thermal stability: the tendency of a compound to resist decomposition when heated
The lower a compound's standard enthalpy of formation, the more stable the compound.
1.39. Module Summary/Assessment
Module 1—Thinking Energy
 Module Summary
Module Summary

© Sascha Corti/shutterstock
We live in a world that is dependent upon energy. In Module 1 you learned about energy and energy transformations.
You considered the following module questions:
- Should energy be given a higher priority when making decisions about society’s future?
- How does society use the energy of chemical changes?
- What are the impacts of energy use on the environment?
- How does society use knowledge of the energy associated with chemical process to promote sustainability?
- In what ways have issues of energy use affected the development of past and present societies?
As you worked through Module 1, you developed an ecotour operating plan. You learned to identify and assess energy sources that society uses in a variety of ways, and you learned to evaluate various materials with respect to the specific heat capacity of each one. You also learned to evaluate fuels with respect to their energy change upon combustion and energy content per mole of fuel combusted.
In Module 2 you will further consider the differences between the energy content of different fuels. You will use this information to evaluate alternative fuels that could be used to fuel vehicles used in your ecotour.
Concept Map or Graphic Organizer
As you worked through Module 1, you may have added information to a concept map or graphic organizer based on the module and lesson questions listed in the Module 1 Concept Organizer. Now is a good time to review the relationships in your concept map or graphic organizer and to try to answer the module and lesson questions.
A sample Module 1 concept map shows one set of possible links between the questions. Remember that this is one possible description only—there are many other correct possibilities. However, if your completed concept map or graphic organizer differs significantly from the sample, you may wish to contact your teacher or to compare your map or organizer with those of other students in your class. This will ensure that your interpretations of lesson materials and your descriptions are accurate.
 Module Assessment
Module Assessment
Ecotour Operations Plan

© Richard A. McGuirk/shutterstock
- In this module you began an operations plan for an ecotour. You considered what activities to offer to tourists and what food and equipment these activities would require. At the completion of this module your plan should include responses to the following activities:
- Choice of Snack Foods (Lesson 2)
- Choice of Sleeping Bag (Lesson 3)
- Recharging Hot Packs (Lesson 4)
- Evaluating Different Fuels (Lesson 5)
- Evaluating Two Liquid Fuels (Lesson 6)
- Reflect on the Big Picture (Lesson 8)
Review your responses, and then select your best four responses for submission to your teacher.
-
Develop an advertising brochure to describe and attract tourists to the tour you are providing. Make sure your brochure explains how your tour is consistent with the principles of ecotourism.
Submit your work to your teacher. Your Module 1 Assessment will also form part of your Unit A Assessment. Please see the Unit A Assessment for more information.
Scoring Guide for Ecotourism Company Brochure
Score |
Criteria |
5 |
The response is a thorough description and explanation of an ecotour. The brochure is attractive and is written using convincing language and style. Correct choices and explicit justifications are made. Detailed explanations are used to identify how the proposed tour is consistent with the principles of ecotourism. |
4 |
The response is an accurate description and explanation of an ecotour. The brochure is attractive and is written using convincing language and style. Correct choices and appropriate justifications are made. Detailed explanations are used to identify how the proposed tour is consistent with the principles of ecotourism. |
3 |
The response is an accurate description of an ecotour. The brochure is attractive and is written using appropriate language and style. Correct choices and supporting information is identified. Explanations are used to identify how the proposed tour is consistent with some of the principles of ecotourism. |
2 |
The response identifies some aspects of an ecotour. The brochure conveys a message with limited impact on the reader. Some correct choices are supported, but with limited explanation and justification. |
1 |
The response identifies an aspect of an ecotour. The brochure fails to convey an appropriate message or tone to the reader. Some relevant information is provided but with insufficient support and organization. |
0 |
The response is incorrect and/or totally off topic. |
1.40. Module Glossary
Module 1—Thinking Energy
Module Glossary
calorimeter: an isolated system in which the chemical system being studied is surrounded by a known quantity of liquid
calorimetry: the technological process of measuring the energy changes of an isolated system
carbohydrate: a carbon compound that contains many hydroxyl groups and has the general chemical formula CnH2nOn (e.g., glucose, C6H12O6)
chemical potential energy: a stored form of energy dependent on the relative positions of particles
endothermic: a process that absorbs energy from its surroundings
enthalpy: the total kinetic and potential energy in a system
enthalpy of reaction: the change in the energy of a chemical system as it proceeds from reactants to products
exothermic: a process that radiates energy to its surroundings
hydrocarbon molecule: a chemical compound containing only hydrogen and carbon atoms
kinetic energy: a form of energy related to the motion of particles
Kinetic energy can involve vibration, rotation, or translation within or of a particle. Kinetic energy changes are often observed as a temperature change in matter.
kinetic molecular theory: a theory that states that small particles that make up a substance are in continuous motion and collide with each other and objects in their path
magnitude: a value that indicates size or quantity
molar enthalpy: a change in enthalpy of a chemical system per mole
quantitative: an observation that uses a numeric value
reference energy state: a reference point at which the potential energy of the elements in their most stable form at SATP is defined as zero
specific heat capacity: a physical property of matter
It is the energy required to raise the temperature of one gram of a substance one degree Celsius.
standard enthalpy of formation: the enthalpy change calculated from the measurements of a formation reaction under standard conditions
thermal conductance: the rate at which thermal energy flows through a substance
thermal stability: the tendency of a compound to resist decomposition when heated
The lower a compound's standard enthalpy of formation, the more stable the compound.