Module 1 Intro
1. Module 1 Intro
1.17. Page 3
Module 1—Energy Flow and the Cycling of Matter
Characteristics of Water
Water is a finite resource in the biosphere, and it can be found in three different states: liquid, solid, and gas. Since water is a finite resource, it must be cycled. There is a lot of water in the biosphere, and it is mostly (97%) found in liquid form. A molecule of water will undergo many phase changes in order to complete a full turn of the hydrologic cycle.
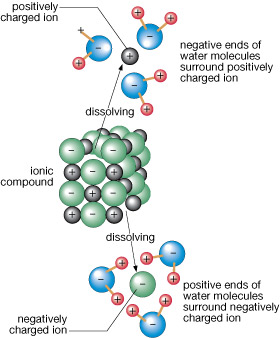
Dissolving substances allow water to carry nutrients to other parts of an ecosystem where they may be used as needed. Keep in mind that water does not discriminate between dissolvable matter! Polar wastes and toxic substances can also be dissolved and transported in water.
Benefits of Hydrogen Bonding
Properties of water that are influenced by hydrogen bonding between water molecules include cohesion, adhesion, and heat capacity.
cohesion: inter-molecular attraction between like-molecules of a substance
Water is strongly cohesive due to the polarity of its molecules.
adhesion: the tendency of unlike molecules to cling together because of attractive forces
Water is adhesive also due to polarity—the negative and positively charged molecules in water attract other ionic molecules.
heat capacity: the amount of heat energy (J) required to change the temperature of one gram of substance by 1°C
Water has a high heat capacity of 4.19 J/g°C.

© basel101658/shutterstock
The description of hydrogen bonding you just read, which describes the attraction between neighbouring water molecules, is often referred to as cohesion within water. The strength of cohesion between water molecules results in water’s high surface tension. As shown in the photograph, the cohesive forces between water molecules support the spider’s weight. Surface tension allows the surface of a body of water to become a habitat for many insects. This increases the biodiversity within aquatic ecosystems.
Adhesion is the attraction of water molecules to other substances. This allows water to be drawn upwards within plant tissues, against the force of gravity, from the roots to the leaves. Once water reaches the leaves, it becomes involved in the process of photosynthesis.
As you may recall from previous science courses, photosynthesis is the primary energy-producing process for ecosystems. Adhesion permits water to be drawn to the leaves of large plants, increasing the proportion of solar energy captured by producers. Capturing solar energy, in turn, greatly increases the energy available for consumers.
Finally, the attractive forces between water molecules have the ability to store thermal energy. Water’s high heat capacity allows large quantities of heat to be absorbed and slowly released back into the surroundings. Having a high heat capacity allows water to moderate the temperature within systems—this helps maintain a relatively constant body temperature or reduce large fluctuations in climate.
 Watch and Listen
Watch and Listen
Water quality affects both producers and consumers within an ecosystem. As you have learned, water is a universal solvent. This makes water an excellent medium in which to carry particles within the biosphere. As mentioned earlier, water is not able to discriminate against particles as either good or bad. Human activities can influence what becomes dissolved in water, affecting water quality.
The Canadian Broadcasting Corporation (CBC) has posted archived audio and television clips from the 1950s to 2000 on its website that chronicle the ongoing effects of human activity to the water quality of the Great Lakes. To access this series of sound bites, search for "CBC" in your search engine. Once you are at the home CBC site, locate the search box in the top-right corner and enter the keyword search "Troubled Waters." The first link should take you to "Troubled Waters: Trouble in the Great Lakes." Pick several clips to view and listen to. What are your thoughts after listening to some of these clips?
The pollution of a lake might be understandable, but what about the quality of the water you drink? Water treatment involves altering the concentration of substances dissolved in water to meet national standards. In some areas of Canada, work is still required to ensure that people have safe drinking water. Return to the CBC home page and enter the keyword search term “slow boil.” Select the first URL. Read the introductory material describing water-quality issues confronting many people who live on First Nations reserves.
Once you have read through the introduction, choose the “Audio” clip about a reserve in Nova Scotia.
 Try This
Try This
TR 1. Canada is a developed country. Is it surprising that some Canadians do not have access to a clean water source? Why do these people not have access to clean water? What should be done about this? Save your response to your course folder.
 Read
Read
Finish reading this section in the textbook from pages 38 to 40. Be sure to read through “Thought Lab 2.1: Water Gains and Losses” on page 38.
 Self-Check
Self-Check
SC 6. What are the similarities between the water budget of a person and a kangaroo rat?
SC 7. Answer questions 4 and 5 on page 39 of the textbook.
 Self-Check Answers
Self-Check Answers
SC 6. In both organisms, water gained = water lost and the most water is lost through urine and evaporation.
SC 7.
- Organisms gain water through eating, metabolism, and drinking.
- Organisms lose water through urine, feces, and evaporation (breathing and sweating).