Module 1 Intro
1. Module 1 Intro
1.25. Page 4
Module 1—Energy Flow and the Cycling of Matter
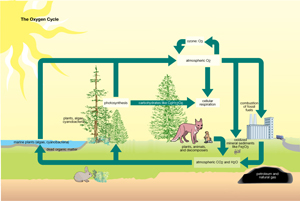
The Oxygen Cycle
Each breath you take involves the essential process of extracting oxygen from the atmosphere. Most living things cannot survive without a source of oxygen. Even aquatic organisms take in dissolved oxygen from the water. Why is oxygen so essential? Oxygen reacts so intensely with other elements in chemical reactions that significant amounts of energy are released. Organisms can then use the energy released in these oxidation reactions.
Given the reactive nature of oxygen, it is natural to wonder why there is any oxygen left in the atmosphere. After all, if oxygen combines so readily with elements like iron to form oxidized mineral sediments, why hasn’t the oxygen all been used up? The answer is photosynthesis.
According to the fossil record, billions of years of photosynthesis by cyanobacteria living in warm, shallow seas created Earth’s current oxygen-rich atmosphere. Modern plants maintain this atmosphere by adding to the reserve of atmospheric oxygen. This atmosphere is balanced by cellular respiration where energy is released from the combustion of food molecules in the presence of oxygen gas.
Connections to the Carbon Cycle
The oxygen cycle has much in common with the carbon cycle—the sinks for carbon are the sources for oxygen, and vice versa. In both cases, the main processes responsible for cycling through ecosystems are photosynthesis and cellular respiration.
Despite these similarities, there are some important differences. Carbon dioxide comprises only about 0.03% of the atmosphere, whereas oxygen accounts for 21%. This means that the dynamic between carbon and oxygen is only a small part of the total oxygen system.
The total oxygen system also involves the cycling of other nutrients, such as sulfur, phosphorus, and nitrogen. To simplify matters, only the connection to carbon is shown in “The Oxygen Cycle” illustration.
When you study the nitrogen cycle, you will see another example of a process that connects to the total oxygen system.
 Try This
Try This
TR 1. Sketch a unified diagram that identifies processes common to both the carbon cycle and the oxygen cycle. Identify the key forms in which carbon and oxygen will occur within both the oxygen cycle and the carbon cycle.
 Self-Check
Self-Check
SC 4. Answer question 10 on page 46 of your textbook.
© KLJ Photographic Ltd/iStockphoto
 Self-Check Answers
Self-Check Answers
SC 4.
10.
-
Deforestation reduces the amount of photosynthesis → less CO2 is removed from the atmosphere by plants, which reduces the amount of carbon and oxygen cycled through the biosphere. Deforestation also reduces the amount of energy available to primary consumers. Debris or leftover biomass will decompose, and carbon dioxide will be released to the atmosphere.
-
Burning fossil fuels releases carbon from slow cycling and quickly increases the amount of carbon in the atmosphere (relatively speaking).
-
Agriculture creates crops—photosynthesis takes in CO2 and provides energy (glucose) to consumers. However, agriculture often relies on deforestation for land, which will increase carbon dioxide as overall photosynthesis decreases (trees remove more carbon dioxide from the air than crops)
 Read
Read
Although the sulfur cycle is not one of the elements that you are required to explain, sulfur is still an important cycled element. Read about this element on page 46 of the textbook, starting at “The Sulfur Cycle.” Also read page 47 of the textbook and page 48 up to “The Nitrogen Cycle.” Acid deposition is a term in this reading that you should know.
 Self-Check
Self-Check
SC 5. Answer question 11 on page 48 of the textbook.
SC 6. Answer question 12 on page 48 of the textbook.
SC 7. Is acid deposition limited to sulfur compounds? Why?
 Self-Check Answers
Self-Check Answers
SC 5.
- Acid deposition is natural and returns sulfur to the soil and the ocean but too much deposition can damage plants and lakes. Nutrients may also be leached from the soil
SC 6.
- Bacteria convert sulfur compounds and add the sulfur back to the soil.
SC 7. No, water molecules can combine with nitrogen, phosphate, and carbon molecules to create acidic solutions.