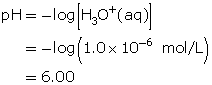Module 5 Intro
1. Module 5 Intro
1.32. Page 3
Module 5—Acids and Bases
 Read
Read
Read “6.3 Acid-Base Indicators” on pages 245 to 247 of your textbook.
As described in your textbook, the different colours of an indicator correspond to the different forms that indicator molecules can have. You may have noticed that the difference between the two forms, HIn and In-, is a hydrogen ion, H+. Can you describe a type of reaction that could be responsible for the loss or gain of an H+ by a substance?
Earlier in this module you wrote chemical equations describing reactions of acids and bases. You may have noticed that during a reaction, acids lose a hydrogen ion and bases gain a hydrogen ion. Indicators are weak acids and bases that will change form (and colour) in response to the solution in which they are dissolved. In basic solutions, litmus will exist in a form represented by the symbol, Lt−, which is blue. In acidic solutions, litmus will exist in a form represented by the symbol HLt, which is pink.
solution changes
So far in this activity you have seen that compounds within tea and red cabbage can act as an acid-base indicator. Many indicators exist. Click on the following link to view a table that lists the indicators that are commonly used in laboratories. A similar version of this table appears in the back cover of your textbook.
Here is a brief explanation of each heading that appears in the Acid-Base Indicators table:
- Indicator: Indicators typically have complicated chemical structures—knowledge of which is not required for the majority of chemistry experiments. As such, for simplicity, the common names are used instead of the chemical names.
- Suggested Abbreviation(s): The complicated chemical structures are represented using a simple abbreviation. Some indicators, such as methyl violet, have only one hydrogen and are capable of only one colour change (yellow to blue). Other indicators, such as thymol blue, are polyprotic and have multiple colours due to the three possible forms for this molecule:
H2Tb (red), HTb– (yellow) , and Tb2–. (blue)
- pH Range: This lists the pH values where the colour of the indicator changes. For example the range for methyl red is 4.8 to 6.0. This means in solutions with a pH below 4.8, methyl red will appear red (HMr); and in solutions with a pH above 6.0, it will appear yellow (Mr-) . The indicator begins to change its form at the first pH value listed in the range, and all the molecules will have been converted to the other form at the second pH value.
- Colour Change as pH Increases: The first colour listed corresponds with the acid form, HIn, of the indicator. The second colour listed corresponds with the basic form, In–.
- Ka: This information is not required in your studies in Chemistry 20.
 Self-Check
Self-Check
SC 1. Use the Acid-Base Indicator table to answer Section 6.3 Questions 1(a), 1(b), 1(d), and 2 on page 247 of your textbook.
SC 2. Two drops of orange IV indicator are added to a solution with a pH of 1.3. What is the colour of the indicator?
SC 3. A beaker containing hydrochloric acid has a hydronium-ion concentration of 7.2 × 10–4 mol/L. Determine the colour if a few drops of the following indicators are added to the acid solution.
- thymol blue
- methyl orange
SC 4. Thymol blue is added to two beakers containing hydrochloric acid. Determine the colour of the indicator for each of the following concentrations.
 Self-Check Answers
Self-Check Answers
SC 1. Section 6.3 Questions 1(a), 1(b), 1(d), and 2 on page 247
- a. pink
b. yellow
d. orange (mixture of molecules that are red and yellow)
- a. less than 4.8
b. greater than 12.0
c. greater than 5.4
d. between 6.0 and 7.6
SC 2. The colour of the orange IV is red for pH values that are less than or equal to 1.4.
SC 3. Determine the pH of the solution.
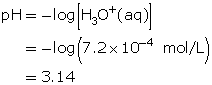
- Thymol blue is yellow for pH values that are greater than or equal to 2.8.
- Methyl orange is red for pH values that are less than or equal to 3.2.
SC 4.
- Determine the pH of the solution.
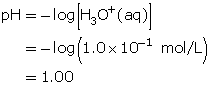
At this pH, thymol blue is red.
- Determine the pH of the solution.